Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Borylstannylation of alkynes with inverse regioselectivity: copper-catalyzed three-component coupling using a masked diboron†
H.
Yoshida
*ab,
Y.
Takemoto
a and
K.
Takaki
a
aDepartment of Applied Chemistry, Graduate School of Engineering, Hiroshima University, Higashi-Hiroshima 739-8527, Japan. E-mail: yhiroto@hiroshima-u.ac.jp; Fax: +81-82-424-5494; Tel: +81-82-424-7724
bACT-C, Japan Science and Technology Agency, Higashi-Hiroshima 739-8527, Japan
First published on 3rd February 2015
Abstract
A variety of terminal alkynes are facilely convertible into cis-boryl(stannyl)alkenes with inverse regioselectivity to those of the previous borylstannylation by the copper-catalyzed three-component reaction using a masked diboron. The synthetic utility of the resulting boryl(stannyl)alkenes has been demonstrated by chemoselective coupling reactions.
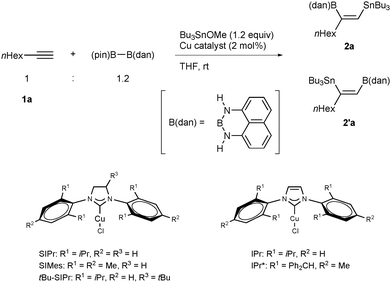
Transition metal-catalyzed dimetallation of alkynes has commanded considerable attention1 because it provides a convenient and direct method for constructing regio- and stereo-defined dimetallated alkenes, whose carbon–metal bonds are utilizable for carbon–carbon bond-forming processes2 to give multisubstituted alkenes, which constitute an important class of biologically and pharmaceutically active molecules. One of the most valuable dimetallation reactions would be borylstannylation, in which the resulting hetero-dimetallic moieties can undergo chemoselective cross-coupling reactions (Suzuki–Miyaura3 and Migita–Kosugi–Stille coupling4) in tandem with high functional group compatibility under controlled reaction conditions. Since the pioneering work reported by Tanaka,5a borylstannylation has hitherto been achieved by direct insertion of alkynes into a B–Sn bond of borylstannanes under palladium catalysis.5 On the other hand, we have recently disclosed a different mode of the borylstannylation by a copper-catalyzed three-component coupling using a diboron and a tin alkoxide.6–8 Irrespective of the catalytic systems and the reaction modes, terminal alkynes exclusively accept the regioselective addition of the boryl group at the terminal carbon and the stannyl group at the internal carbon in a cis fashion to give (Z)-1-boryl-2-stannyl-1-alkenes (Scheme 1), and thus we have focused our attention on the reversal of regioselectivity, which increases structural diversity of vic-boryl(stannyl)alkenes and thereby broadens the synthetic utility of the borylstannylation. Herein we report that the use of a masked diboron9 in the copper-catalyzed three-component borylstannylation of terminal alkynes completely inverts the regioselectivity, and that this method provides a convenient and direct access to unprecedented hetero-dimetallated alkenes having masked boryl and stannyl moieties.10
First we conducted the reaction of 1-octyne (1a) with a masked diboron ((pin)B–B(dan), pin: pinacolato, dan: naphthalene-1,8-diaminato11) and tributyltin methoxide in THF at room temperature in the presence of an N-heterocyclic carbene (NHC)-coordinated copper complex ((SIPr)CuCl), and found that the cis-borylstannylation took place with regioselectivity inverse to those of the previous borylstannylation (74% yield, 2a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2′a = 96
2′a = 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4), leading to the introduction of the boryl group at the internal carbon and the stannyl group at the terminal carbon (Table 1, entry 1). It is noteworthy that the B(dan) moiety was solely installed in the product, and a borylstannylation product having the B(pin) moiety was not formed at all. The regioselectivity for the formation of 2a was generally high with bulky ligands (SIMes, tBu-SIPr, IPr, IPr*12 and P(tBu)3) (entries 2–6), whereas the use of triphenylphosphine ((PPh3)3CuCl) led to the formation of regioisomeric mixtures (2a
4), leading to the introduction of the boryl group at the internal carbon and the stannyl group at the terminal carbon (Table 1, entry 1). It is noteworthy that the B(dan) moiety was solely installed in the product, and a borylstannylation product having the B(pin) moiety was not formed at all. The regioselectivity for the formation of 2a was generally high with bulky ligands (SIMes, tBu-SIPr, IPr, IPr*12 and P(tBu)3) (entries 2–6), whereas the use of triphenylphosphine ((PPh3)3CuCl) led to the formation of regioisomeric mixtures (2a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2′a = 44
2′a = 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 56, entry 7). In addition, the reaction with PCy3, used for the previous borylstannylation with bis(pinacolato)diboron,6a also afforded 2a preferentially (2a
56, entry 7). In addition, the reaction with PCy3, used for the previous borylstannylation with bis(pinacolato)diboron,6a also afforded 2a preferentially (2a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2′a = 84
2′a = 84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16, entry 8), which reveals that the choice of a diboron as well as a ligand is the key to achieving the present regioselectivity.13 Since an increase in the amount of tributyltin methoxide resulted in an increase in the yield with the highest regioselectivity (86% yield, entry 9), we selected the conditions for further studies.14
16, entry 8), which reveals that the choice of a diboron as well as a ligand is the key to achieving the present regioselectivity.13 Since an increase in the amount of tributyltin methoxide resulted in an increase in the yield with the highest regioselectivity (86% yield, entry 9), we selected the conditions for further studies.14
| Entry | Cu catalyst | Time (h) | Yieldb (%) |
2a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2′ac 2′ac |
|---|---|---|---|---|
| a General procedure: 1a (0.30 mmol, 1 equiv.), (pin)B–B(dan) (0.36 mmol, 1.2 equiv.), Bu3SnOMe (0.36 mmol, 1.2 equiv.), Cu catalyst (6.0 μmol, 2 mol%), THF (1 mL). b Isolated yield. c Determined using 1H NMR. d Ligand = 4 mol%. e Bu3SnOMe = 2 equiv. | ||||
| 1 | (SIPr)CuCl | 7 | 74 | 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
| 2 | (SIMes)CuCl | 2 | 81 | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
| 3 | (tBu-SIPr)CuCl | 20 | 69 | 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
| 4 | (IPr)CuCl | 5 | 75 | 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
| 5 | (IPr*)CuCl | 11 | 80 | 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
| 6d | P(tBu)3, CuCl | 2 | 81 | 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
| 7 | (PPh3)3CuCl | 48 | 75 | 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 56 56 |
| 8d | PCy3, CuCl | 14 | 60 | 84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 16 |
| 9e | (SIPr)CuCl | 10 | 86 | 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
With the optimum conditions in hand (Table 1, entry 9), the substrate scope of alkynes was next investigated (Table 2). Such aliphatic terminal alkynes as 1-hexyne (1b), 4-methyl-1-pentyne (1c) and 4-phenyl-1-butyne (1d) also underwent the borylstannylation with high degrees of regioselectivity to give 2b, 2c and 2d in 78, 81 and 74% yield (entries 1–3). The functional group compatibility of the reaction was sufficiently high, and thus a C–Br bond15 in 1e and a cyano group in 1f remained intact throughout the reaction (entries 4 and 5). The present regioselectivity was also observed by using enyne (1g) and phenylacetylene (1h) (entries 6 and 7), and furthermore the reaction of propargyl ethers (1i and 1j) or a THP-protected propargyl alcohol (1k) resulted in the exclusive formation of 2i–2k (entries 8–10). In addition, propargyl amine (1l) and trimethylsilylacetylene (1m) accepted the addition of the B(dan) moiety at their internal carbon with perfect regioselectivity (entries 11 and 12).16 The versatility of the borylstannylation was further expanded by application to 1,7-octadiyne17 (1n) and allenes18 (3a and 3b): both of the triple bonds were convertible regioselectively into the borylstannylalkenes in the former case, and the regio- and stereoselective reaction proceeded to provide (Z)-1-stannyl-2-boryl-2-alkenes (4a and 4b) as the single product, although the regioselectivity is similar to that of the previous borylstannylation with bis(pinacolato)diboron6b in the latter case (Scheme 2).
| Entry | R | Yieldb (%) |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2′c 2′c |
Products |
|---|---|---|---|---|
| a General procedure: 1 (0.30 mmol, 1 equiv.), (pin)B–B(dan) (0.36 mmol, 1.2 equiv.), Bu3SnOMe (0.60 mmol, 2 equiv.), (SIPr)CuCl (6.0 μmol, 2 mol%), THF (1 mL). b Isolated yield. c Determined using 1H NMR. | ||||
| 1 | nBu (1b) | 78 | 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
2b, 2′b |
| 2 | iBu (1c) | 81 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2c, 2′c |
| 3 | Ph(CH2)2 (1d) | 74 | 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
2d, 2′d |
| 4 | Br(CH2)2 (1e) | 87 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2e, 2′e |
| 5 | NC(CH2)3 (1f) | 79 | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
2f, 2′f |
| 6 | 1-Cyclohexenyl (1g) | 81 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2g, 2′g |
| 7 | Ph (1h) | 73 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2h, 2′h |
| 8 | MeOCH2 (1i) | 66 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2i |
| 9 | BnOCH2 (1j) | 66 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2j |
| 10 | THPOCH2 (1k) | 66 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2k |
| 11 | Et2NCH2 (1l) | 69 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2l |
| 12 | Me3Si (1m) | 75 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2m |
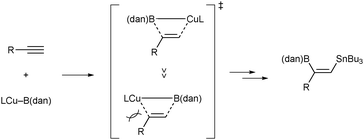
Similarly to the previous copper-catalyzed borylstannylation with bis(pinacolato)diboron,6 generation of a borylcopper species, Cu–B(dan), from Cu–OMe and a masked diboron commences the reaction (Scheme 3, step A). Subsequent insertion of an alkyne into the Cu–B(dan) bond, which produces a β-borylalkenylcopper species (borylcupration, step B),19 followed by capturing with tin methoxide furnishes the product (step C).20 The formation of Cu–B(dan) (vs. Cu–B(pin)) can be rationally explained by selective interaction between the Lewis acidic B(pin) moiety of (pin)B–B(dan) and the methoxy moiety of Cu–OMe in step A, leading to the exclusive introduction of the masked boryl moiety across the triple bond of alkynes. The orientation of a borylcopper species in the borylcupration step entirely governs the regiochemical outcome of the borylstannylation (Scheme 4), and the mode of the borylcupration with Cu–B(dan) would simply be controlled by steric repulsion between a substituent on alkynes and a bulkier copper moiety as was the case with the hydroboration.10a Hence, the B(dan) moiety is solely installed into the internal carbon of terminal alkynes,21,22 which results in the inverse regioselectivity in the present borylstannylation.
Synthetic utility of the boryl(stannyl)alkenes was demonstrated by the chemoselective cross-coupling: a C–Sn bond of 2i was solely convertible into a C–C bond by the palladium-catalyzed Migita–Kosugi–Stille reaction to provide an 82% yield of 5 with a masked boryl moiety remaining intact (Scheme 5). Furthermore, the masking enabled the copper-mediated oxidative homocoupling to take place at the C–Sn bond selectively, affording 1,4-diboryl-1,3-butadienes (6–8) stereoretentively in high yield. Unmasking of the resulting 1,4-diboryl-1,3-butadiene, followed by the Suzuki–Miyaura reaction with 4-iodotoluene furnished 1,1,4,4-tetraarylbutadiene 9.
In conclusion, we have disclosed that the borylstannylation of terminal alkynes proceeds with inverse regioselectivity by the copper-catalyzed three-component reaction using a masked diboron, which gives us a convenient and potent approach to diverse cis-boryl(stannyl)alkenes bearing the masked boryl moiety at the internal carbons. Moreover, the synthetic versatility of the resulting boryl(stannyl)alkenes has been shown by the chemoselective coupling reactions depending on the difference in the reactivity between the masked boryl and the stannyl moieties. Further studies on copper-catalyzed borylation reactions using a masked diboron as well as on the details of the mechanism are in progress.
Notes and references
- For reviews, see: (a) I. Beletskaya and C. Moberg, Chem. Rev., 1999, 99, 3435 CrossRef CAS PubMed; (b) M. Suginome and Y. Ito, Chem. Rev., 2000, 100, 3221 CrossRef CAS PubMed; (c) I. Beletskaya and C. Moberg, Chem. Rev., 2006, 106, 2320 CrossRef CAS PubMed.
- Metal-Catalyzed Cross-Coupling Reactions, ed. A. de Meijere and F. Diederich, Wiley-VHC, Weinheim, 2004 Search PubMed.
- (a) N. Miyaura and A. Suzuki, Chem. Rev., 1995, 95, 2457 CrossRef CAS; (b) N. Miyaura, Top. Curr. Chem., 2002, 219, 11 CrossRef CAS.
- V. Farina, V. Krishnamurthy and W. J. Scott, Org. React., 1997, 50, 1 CAS.
- (a) S. Onozawa, Y. Hatanaka, T. Sakakura, S. Shimada and M. Tanaka, Organometallics, 1996, 15, 5450 CrossRef CAS; (b) S. Onozawa, Y. Hatanaka, N. Choi and M. Tanaka, Organometallics, 1997, 16, 5389 CrossRef CAS; (c) L. Weber, H. B. Wartig, H.-S. Stammler, A. Stammler and B. Neumann, Organometallics, 2000, 19, 2891 CrossRef CAS; (d) R. R. Singidi and T. V. RajanBabu, Org. Lett., 2010, 12, 2622 CrossRef CAS PubMed.
- (a) Y. Takemoto, H. Yoshida and K. Takaki, Chem. – Eur. J., 2012, 18, 14841 CrossRef CAS PubMed; (b) Y. Takemoto, H. Yoshida and K. Takaki, Synthesis, 2014, 3024 CAS.
- We have also reported copper-catalyzed borylation and stannylation reactions of alkynes and alkenes. See: (a) H. Yoshida, S. Kawashima, Y. Takemoto, K. Okada, J. Ohshita and K. Takaki, Angew. Chem., Int. Ed., 2012, 51, 235 CrossRef CAS PubMed; (b) H. Yoshida, I. Kageyuki and K. Takaki, Org. Lett., 2013, 15, 952 CrossRef CAS PubMed; (c) H. Yoshida, A. Shinke and K. Takaki, Chem. Commun., 2013, 49, 11671 RSC; (d) I. Kageyuki, H. Yoshida and K. Takaki, Synthesis, 2014, 1924 Search PubMed.
- For a review on copper-catalyzed borylation reactions of alkynes with diborons, see: (a) J. Yun, Asian J. Org. Chem., 2013, 2, 1016 CrossRef CAS; (b) T. Fujihara, K. Semba, J. Terao and Y. Tsuji, Catal. Sci. Technol., 2014, 4, 1699 RSC.
- (a) N. Iwadate and M. Suginome, J. Am. Chem. Soc., 2010, 132, 2548 CrossRef CAS PubMed; (b) N. Miralles, J. Cid, A. B. Cuenca, J. J. Carbó and E. Fernández, Chem. Commun., 2015, 51, 1693 RSC.
- We have recently reported copper-catalyzed hydroboration of alkynes and alkenes with a masked diboron. See: (a) H. Yoshida, Y. Takemoto and K. Takaki, Chem. Commun., 2014, 50, 8299 RSC; (b) H. Yoshida, Y. Takemoto and K. Takaki, Asian J. Org. Chem., 2014, 3, 1204 CrossRef CAS.
- For boron-masking strategy with 1,8-diaminonaphthalene, see: (a) H. Noguchi, K. Hojo and M. Suginome, J. Am. Chem. Soc., 2007, 129, 758 CrossRef CAS PubMed; (b) H. Noguchi, T. Shioda, C.-M. Chou and M. Suginome, Org. Lett., 2008, 10, 377 CrossRef CAS PubMed; (c) N. Iwadate and M. Suginome, Org. Lett., 2009, 11, 1899 CrossRef CAS PubMed; (d) N. Iwadate and M. Suginome, Chem. Lett., 2010, 39, 558 CrossRef CAS.
- (a) G. Berthon-Gelloz, M. A. Siegler, A. L. Spek, B. Tinant, J. N. H. Reek and I. E. Markó, Dalton Trans., 2010, 39, 1444 RSC; (b) J. Balogh, A. M. Z. Slawin and S. P. Nolan, Organometallics, 2012, 31, 3259 CrossRef CAS.
- A boryl moiety is selectively installed into a terminal carbon of 1-octyne in the borylstannylation with bis(pinacolato)diboron under the Cu–PCy3 catalysis, which is in marked contrast to the results described herein. See ref. 6a.
- The alkyne (1a) had already been consumed at the time indicated in Table 1, and thus the yields would not change if the reactions are left longer.
- For copper-catalyzed borylation of alkyl halides with a diboron, see: (a) C.-T. Yang, Z.-Q. Zhang, H. Tajuddin, C.-C. Wu, J. Liang, J.-H. Liu, Y. Fu, M. Czyzewska, P. G. Steel, T. B. Marder and L. Liu, Angew. Chem., Int. Ed., 2012, 51, 528 CrossRef CAS PubMed; (b) H. Ito and K. Kubota, Org. Lett., 2012, 14, 890 CrossRef CAS PubMed.
- The reaction of ethyl propiolate did not produce the borylstannylation product at all.
- For palladium-catalyzed cyclizative borylstannylation of diynes with a borylstannane, see: (a) R. R. Singidi and T. V. RajanBabu, Org. Lett., 2008, 10, 3351 CrossRef CAS PubMed; (b) R. R. Singidi, A. M. Kutney, J. C. Gallucci and T. V. RajanBabu, J. Am. Chem. Soc., 2010, 132, 13078 CrossRef CAS PubMed . See also ref. 5b.
- Palladium-catalyzed borylstannylation of an allene with a borylstannane is accompanied by dimerization of an allene. See: S. Onozawa, Y. Hatanaka and M. Tanaka, Chem. Commun., 1999, 1863 RSC.
- Generation of a borylcopper species (step A) and insertion of an alkyne into the Cu–B bond (step B) have been widely accepted as fundamental elementary steps in the copper-catalyzed borylation reactions of alkynes with diborons. See ref. 8.
- We have already verified that an alkenylcopper species is facilely captured with a tin methoxide to produce an alkenylstannane. See ref. 6a.
- Installation of a B(pin) moiety into a terminal carbon of terminal alkynes is commonly observed in copper-catalyzed hydroboration with (pin)B–B(pin). See: (a) J.-E. Lee, J. Kwon and J. Yun, Chem. Commun., 2008, 733 RSC; (b) Y. Lee, H. Jang and A. H. Hoveyda, J. Am. Chem. Soc., 2009, 131, 18234 CrossRef CAS PubMed; (c) H. Jang, A. R. Zhugralin, Y. Lee and A. H. Hoveyda, J. Am. Chem. Soc., 2011, 133, 7859 CrossRef CAS PubMed.
- α-Selective hydroboration of terminal alkynes with (pin)B–B(pin) proceeds in the presence of a copper catalyst coordinated by a sterically demanding ligand (P(tBu)3, SIMes or SIPr), however, the substrate scope is limited to propargyl-functionalized and electron-deficient aryl ones, being in marked contrast to the results described herein. See: A. L. Moure, P. Mauleón, R. G. Arrayás and J. C. Carretero, Org. Lett., 2013, 15, 2054 CrossRef CAS PubMed . See also ref. 21c.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and characterization data. See DOI: 10.1039/c5cc00439j |
| This journal is © The Royal Society of Chemistry 2015 |