Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Palladium(II)-catalysed ortho-arylation of N-benzylpiperidines†
Peng Wen
Tan
,
Maxwell
Haughey
and
Darren J.
Dixon
*
Department of Chemistry, Chemistry Research Laboratory, University of Oxford, 12 Mansfield Road, Oxford, OX1 3TA, UK. E-mail: darren.dixon@chem.ox.ac.uk
First published on 5th February 2015
Abstract
PdII-catalysed ortho-arylation of benzylic heterocycles with arylboronic acid pinacol esters (Ar-BPin) via directed C–H bond activation to generate the desired biaryl products is reported. This methodology is efficient and applicable to a wide range of functionalised Ar-BPin and benzylic heterocycles, allowing the direct synthesis of important biaryl motifs in modest to good yield.
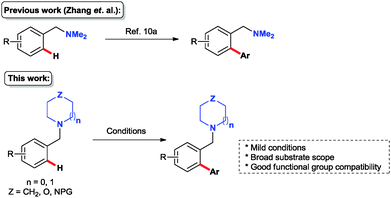
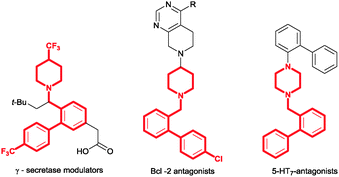
Cross-coupling reactions of aromatic compounds constitute one of the most versatile entries to compounds possessing a biaryl motif.1 Such transformations are usually performed by traditional cross coupling reactions of organohalides or pseudohalides with organometallic reagents.2 However, prefunctionalisation of substrates to form specific organohalides for cross coupling can be difficult and this has accordingly led many research groups to develop more direct routes to biaryl scaffolds.3 Over the last decade, a variety of direct, chelation-assisted, C–H activation on arenes, mainly via [Pd],4 [Rh]5 and [Ru]6 catalysis, has been developed using a wide range of directing groups (DGs) such as amides, imines, oximes, ketones and other N-heterocycles. Expanding the scope of DGs to include simple amine derivatives that are commonly found in natural products and pharmaceutical compounds is highly desirable. In 2006, Daugulis and co-workers reported an ortho arylation of unsubstituted benzylamines directed by the free amine with iodobenzene under Pd catalysis.7 Meanwhile, Shi et al. demonstrated that N,N-di-alkyl amine is also an effective directing group for ortho-olefination8 and -carbonylation.9 More recently, Zhang and co-workers10a developed a Pd-catalysed ortho-arylation of N,N-dimethylbenzylamines with iodobenzene (Fig. 1).10 Inspired by these findings we envisaged that saturated N-containing heterocycles could also serve as efficient DGs for C(sp2)–H arylation; this would expand the range of DGs in the field (Fig. 1) and the new chemistry could be applied directly to the construction of biologically relevant motifs such as those present, for example, in known Bcl-2 antagonists,11 γ-secretase modulators12 and 5-HT7-antagonists13 (Fig. 2). Herein we report a PdII-catalysed ortho-arylation of a range of benzylic heterocycles with arylboronic acid pinacol esters leading directly to the biaryl product in one step.
Initially we focused on the piperidine moiety as a potentially useful directing group for C(sp2)–H activation/arylation. Inspired by Yu's pioneering work,14 a preliminary experiment was conducted on the model substrate 1-(2-methylbenzyl)piperidine 1a and phenylboronic acid pinacol ester 2a in the presence of catalytic Pd(OAc)2, and Ag2CO3, Na2CO3 and 1,4-benzoquinone at 100 °C (Table 1). Encouragingly, ortho-arylated product 3a was observed in 55% NMR yield (entry 1).
| Entrya | Catalyst (mol%) | Oxidant (equiv.) | Base (equiv.) | Yieldc (%) |
|---|---|---|---|---|
| a Reaction condition: 1a (0.2 mmol), 2b (0.28 mmol), Pd catalyst, base, oxidant, BQ (0.5 equiv.), DMSO (5 μL), H2O (20 μL) in t-amylOH (1 mL), T = 100 °C, 18 h. b Similar results for PdCl2(PPh3)2 and PdCl2(CH3CN)2. c 1H NMR yield with internal standard (CH2Br2), in parenthesis isolated yield. | ||||
| 1 | Pd(OAc)2 (10) | Ag2CO3 (2.0) | Na2CO3 (6.0) | 55 |
| 2 | Pd(OAc)2 (10) | Ag2CO3 (2.0) | KF (6.0) | 25 |
| 3 | Pd(OAc)2 (10) | Ag2CO3 (2.0) | K2HPO4 (6.0) | 33 |
| 4 | Pd(OAc)2 (10) | Ag2CO3 (1.5) | NaHCO3 (3.0) | 82 |
| 5b | PdCl2 (10) | Ag2CO3 (1.5) | NaHCO3 (3.0) | — |
| 6 | Pd(OAc)2 (5) | Ag2CO3 (1.5) | NaHCO3 (3.0) | 43 |
| 7 | Pd(OAc)2 (10) | Cu(OAc)2 (1.5) | NaHCO3 (3.0) | 11 |
| 8 | Pd(OAc)2 (10) | CuF2 (1.5) | NaHCO3 (3.0) | 2 |
| 9 | Pd(OAc)2 (10) | Ag2CO3 (2.0) | NaHCO3 (4.0) | 88 (81) |
| 10 | Pd(OAc)2 (10) | AgOAc (2.0) | NaHCO3 (4.0) | 81 |
Further optimisation was then carried out, starting with an investigation of the performance of different bases. Potassium bases, KF and K2HPO4, resulted in a reduced yield (entries 2 & 3). Pleasingly, however, NaHCO3 was found to be optimal for this transformation; full conversion of 1a to the arylated product 3a was observed after 18 hours (entry 4). Replacement of Pd(OAc)2 with other Pd sources such as PdCl2, PdCl2(PPh3)2 and PdCl2(CH3CN)2 did not result in the formation of any arylated product (entry 5). Decreasing the catalyst loading to 5 mol% resulted in a decrease in yield and therefore, 10 mol% of catalyst was deemed necessary (entry 6). Other oxidants such as Cu(OAc)2 and CuF2, were found to give inferior yields (entries 7 & 8) and although AgOAc was found to perform reasonably well (entry 10), Ag2CO3 proved optimal (entry 9). 1,4-Benzoquinone was also essential as a promoter; in its absence no reaction was observed in agreement with literature findings relating to its importance in the reductive elimination step.14b,15 A decline in yield was also observed without the addition of H2O and DMSO (see the ESI†). t-Amyl alcohol was found to be the most suitable solvent for this transformation, followed by 1,4-dioxane and MeCN. Other aryl boronic acid derivatives were investigated, but arylboronic acid pinacol esters, were by far the best in this reaction (see the ESI†).
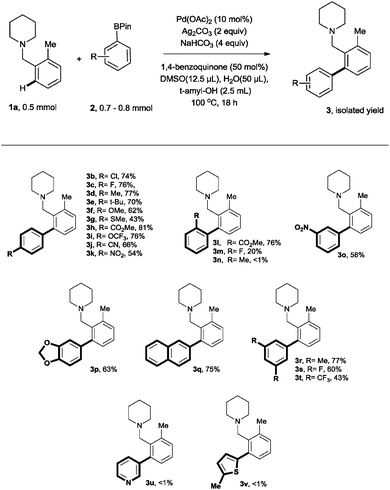
After attaining the optimised conditions, the scope of this methodology was assessed using different functionalized arylboronic acid pinacol esters (Ar-BPins) with 1-(2-methylbenzyl)piperidine (Scheme 1). Generally, a wide range of para-substituted Ar-BPin substrates was tolerated and the corresponding biaryl products were obtained in modest16 to good yields. Ar-BPins substituted with functional groups, such as cyano, nitro and fluorine were suitable substrates under these reaction conditions affording the desired products in respectable yields. Chlorine substituents on Ar-BPin were also well-tolerated, giving rise to biaryl products poised for further transformations. In the case of ortho-substituted Ar-BPin substrates, the reaction worked well using the ester 2l affording the biaryl product 3l in a yield of 76% but, unfortunately, poor conversions were obtained for other substituents such as fluoro (3m) and methyl (3n). This methodology was also amenable to meta-substituted and 3,5-disubstituted Ar-Bpin derivatives, giving rise to the corresponding products in 43–77% yield. In agreement with related studies14f heteroarylboronates that contained pyridine-(3u), thiophene-(3v) were unreactive under these conditions. Similarly, under the optimised conditions attempted ortho alkylation using cyclohexylboronic acid pinacol ester failed to yield any desired product.
The scope with respect to the substituents on the aromatic ring of the benzylpiperidine in the reaction with phenylboronic acid pinacol ester, was then examined. As presented in Scheme 2, ortho-substituted substrates possessing electron withdrawing (F and CF3) and the electron donating (OMe) substituents performed well, with products being afforded in 44–73% yield. meta substituted substrates with sterically bulky electron withdrawing substituents (Br and CF3) underwent monoarylation selectively at the less hindered ortho position, presumably due to steric effects, and afforded the products 4d and 4e in 22–28% yield. However, for less bulky electron withdrawing groups such as fluorine, products of both mono- and di-arylation, 4g and 4g′, were observed in 14% and 35% yield respectively. Notably, for an electron donating substituent at the meta position (OMe), in addition to affording the major monoarylated product 4f (27%), diarylated product 4f′ was also isolated in 12% yield. The reaction conditions were also compatible with para-substituted substrates affording a mixture of mono and di arylated products. Electron donating substituents (OMe) at the para position increased reactivity compared to the electron withdrawing groups and the di-arylated compounds 4h′ and 4i′ were obtained as the major products. Naphthyl (4j) and tri-fluorophenyl (4k) derivatives were also well-tolerated.
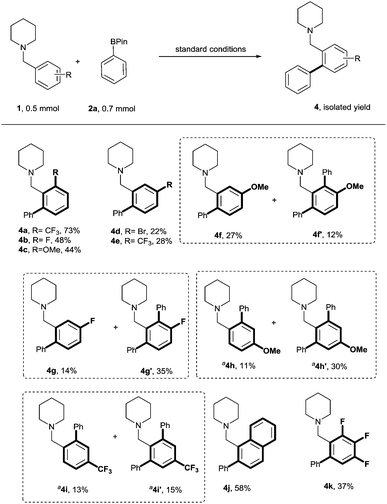 | ||
| Scheme 2 C(sp2)–H cross-coupling of phenylboronic acid pinacol ester with different functionalised benzylpiperidines. a Reaction was carried out with 2a (1.1 mmol) for 24 h. | ||
After exploring arylations directed by the piperidine moiety, we next investigated other possible saturated nitrogen-containing heterocyclic derivatives that could serve as effective directing groups for this transformation. Accordingly, the benzylic heterocycles 5a–f were subjected to our previously optimised ortho-phenylation conditions. (Scheme 3) Gratifyingly, morpholine (5a) and piperazine (5b and 5c) scaffolds worked well, affording the corresponding arylated products in good yield. Reactions with benzylic heterocycles of different ring size was also shown to be successful albeit the yield obtained was low under the standard conditions and no further optimisation was carried out to improve the yield.
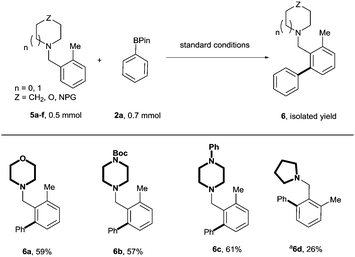 | ||
| Scheme 3 C(sp2)–H cross-coupling of phenylboronic acid pinacol ester with functionalised benzylic heterocycles. a Reaction was carried out with 2a (1.1 mmol) for 24 h. | ||
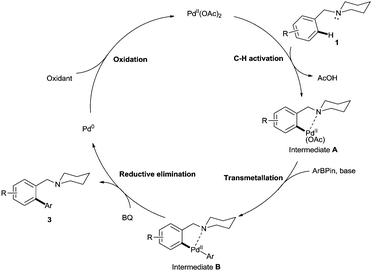
In line with previous studies,14 we propose this C–H activation cross-coupling reaction proceeds via a Pd(II)/Pd(0) catalytic cycle (Fig. 3). The palladium species activates the ortho C–H of 1, forming a 5-membered palladacycle intermediate A. Subsequently, addition of base is necessary for the transmetallation of Ar-Bpin to proceed to generate intermediate B. 1,4-benzoquinone then facilitates reductive elimination of the biaryl product 3 forming the Pd(0) species.14b,15 Reoxidation of Pd(0) by an oxidant, in this case Ag2CO3, is necessary for regeneration of the active Pd(II) species for further turnovers.
In conclusion, we have demonstrated that a range of saturated nitrogen-containing heterocycles attached via the nitrogen atom to benzylic substrates serve as effective directing groups for palladium catalysed C(sp2)–H activation/arylation. Under palladium catalysis, the coupling of a range of arylboronic acid pinocol esters to the ortho position of N-benzylated pyrrolidines, piperidines, morpholine and piperazine substrates giving direct access, under relatively mild reaction conditions, to important biaryls motifs, was demonstrated. Investigations to develop the related direct ortho alkylation reaction are ongoing and our findings will be reported in due course.
The authors acknowledge the University of Oxford and the Agency of Science, Technology and Research (A*STAR) Singapore for a predoctoral fellowship. We also thank Dr Jayasree Seayad and Dr Vaibhav Mehta for their invaluable suggestions.
Notes and references
- (a) Cross-coupling reactions in organic synthesis, Themed issue, , Chem. Soc. Rev., 2011, 40, 4977–5208 Search PubMed and references therein; (b) Handbook of C–H Transformations, ed. G. Dyker, Wiley-VCH, Weinheim, 2005 Search PubMed; (c) J. F. Hartwig, Organotransition Metal Chemistry, University Science Books, 2009 Search PubMed.
- (a) C. C. C. J. Seecchurn, M. O. Kitching, T. J. Calacot and V. Snieckus, Angew. Chem., Int. Ed., 2012, 51, 5062–5085 CrossRef PubMed; (b) Modern Arylation Methods, ed. L. Ackermann, Wiley-VCH, Weinheim, 2009 Search PubMed.
- (a) C–H functionalization in organic synthesis, Themed issue, , Chem. Soc. Rev., 2011, 40, 1845–2040 RSC; (b) C–H functionalization, Themed issue, , Acc. Chem. Res., 2012, 45, 777–958 CrossRef PubMed and references cited therein; (c) J. Wencel-Delord, T. Droge, F. Liu and F. Glorius, Chem. Soc. Rev., 2011, 40, 4740–4761 RSC; (d) Directed C–H Functionalisation, Themed issue, , Adv. Synth. Catal., 2014, 356, 1381–1644 CrossRef , and references therein; (e) J. Yamaguchi, A. D. Yamaguchi and K. Itami, Angew. Chem., Int. Ed., 2012, 51, 8960–9009 CrossRef CAS PubMed.
- (a) R. Giri, B.-F. Shi, K. M. Engle, N. Maugel and J.-Q. Yu, Chem. Soc. Rev., 2009, 38, 3242–3272 RSC; (b) A. J. Hickman and M. S. Sanford, Nature, 2012, 484, 177–185 CrossRef CAS PubMed; (c) J. Feng, G. Lu, M. Lv and C. Cai, Synlett, 2013, 2153–2159 CAS; (d) C. K. Seigerman, T. M. Micyus, S. R. Neufeldt and M. S. Sanford, Tetrahedron, 2013, 69, 5580–5587 CrossRef CAS PubMed; (e) Z.-J. Du, J. Guan, G.-J. Wu, P. Xu, L.-X. Gao and F.-S. Han, J. Am. Chem. Soc., 2014, 137, 632–635 CrossRef PubMed.
- (a) X.-S. Zhang, K. Chen and Z.-J. Shi, Chem. Sci., 2014, 5, 2146–2159 RSC; (b) N. Kuhl, N. Schrçder and F. Glorius, Adv. Synth. Catal., 2014, 356, 1443–1460 CrossRef CAS; (c) G. Song, F. Wang and X. Li, Chem. Soc. Rev., 2012, 41, 3651–3678 RSC; (d) T. Satoh and M. Miura, Chem. – Eur. J., 2010, 16, 11212–11222 CrossRef CAS PubMed.
- (a) S. I. Kozhushkov and L. Ackermann, Chem. Sci., 2013, 4, 886–896 RSC; (b) P. B. Arockiam, C. Bruneau and P. H. Dixneuf, Chem. Rev., 2012, 112, 5879–5918 CrossRef CAS PubMed; (c) L. Ackermann and R. Vicente, Top. Curr. Chem., 2010, 292, 211–229 CrossRef CAS.
- (a) A. Lazareva and O. Daugulis, Org. Lett., 2006, 8, 5211–5213 CrossRef CAS PubMed . For free amine directed C(sp2)–H activation: ; (b) Z. Liang, L. Ju, Y. Xie, L. Huang and Y. Zhang, Chem. – Eur. J., 2012, 18, 15816–15821 CrossRef CAS PubMed; (c) Z. Liang, J. Yao, K. Wang, H. Li and Y. Zhang, Chem. – Eur. J., 2013, 19, 16825–16831 CrossRef CAS PubMed; (d) Z. Liang, R. Feng, H. Yin and Y. Zhang, Org. Lett., 2013, 15, 4544–4547 CrossRef CAS PubMed.
- (a) G. Cai, Y. Fu, Y. Li, X. Wan and Z.-J. Shi, J. Am. Chem. Soc., 2007, 129, 7666–7673 CrossRef CAS PubMed; (b) For early report see: D. M. Grove, G. van Koten and H. J. C. Ubbels, J. Am. Chem. Soc., 1982, 104, 4285–4286 CrossRef CAS.
- H. Li, G.-X. Cai and Z.-J. Shi, Dalton Trans., 2010, 39, 10442–10446 RSC.
- (a) R. Feng, J. Yao, Z. Liang, Z. Liu and Y. Zhang, J. Org. Chem., 2013, 78, 3688–3696 CrossRef CAS PubMed; (b) D.-W. Gao, Y.-C. Shi, Q. Gu, Z.-L. Zhao and S.-L. You, J. Am. Chem. Soc., 2013, 135, 86–89 CrossRef CAS PubMed; (c) For Ru(0)-catalysed ortho-silylation see: F. Kakiuchi, K. Igi, M. Matsumoto, T. Hayamizu, N. Chatani and S. Murai, Chem. Lett., 2002, 396–397 CrossRef CAS; (d) For dehydrogenative annulation see: H. Zhang, X. Cui, X. Yao, H. Wang, J. Zhang and Y. Wu, Org. Lett., 2012, 14, 3012–3015 CrossRef CAS PubMed; (e) For Ir-catalysed borylation see: A. J. Roering, L. V. A. Hale, P. A. Squier, M. A. Ringgold, E. R. Wiederspan and T. B. Clark, Org. Lett., 2012, 14, 3558–3561 CrossRef CAS PubMed.
- B. B. Toure, K. Miller-Moslin, N. Yusuff, L. Perez, M. Dore, C. Joud, W. Michael, L. DiPietro, S. van der Plas, M. McEwan, F. Lenoir, M. Hoe, R. Karki, C. Springer, J. Sullivan, K. Levine, C. Fiorilla, X. Xie, R. Kulathila, K. Herlihy, D. Porter and M. Vaisser, ACS Med. Chem. Lett., 2013, 4, 186–190 CrossRef CAS PubMed.
- Z. Xin, H. Peng, A. Zhang, T. Talreja, G. Kumaravel, L. Xu, E. Rohde, M.-Y. Jung, M. N. Shackett, D. Kocisko, S. Chollate, A. W. Dunah, P. A. Snodgrass-Belt, A. Moore, A. G. Taveras, K. J. Rhodes and R. H. Scannevin, Bioorg. Med. Chem. Lett., 2011, 21, 7277–7280 CrossRef CAS PubMed.
- H. Choo, Y.-J. Kim, J. Kim and M. Y. Yeom, US pat., 2014/0228568 A1, 2014 Search PubMed.
- (a) R. Giri, N. Maugel, J.-J. Li, D.-H. Wang, S. P. Breazzano, L. B. Saunders and J.-Q. Yu, J. Am. Chem. Soc., 2007, 129, 3510–3511 CrossRef CAS PubMed; (b) M. Wasa, K. S. L. Chan and J.-Q. Yu, Chem. Lett., 2011, 40, 1004–1006 CrossRef CAS PubMed; (c) K. M. Engle, P. S. Thuy-Boun, M. Dang and J.-Q. Yu, J. Am. Chem. Soc., 2011, 133, 18183–18193 CrossRef CAS PubMed; (d) M. Wasa, K. M. Engle, D. W. Lin, E. J. Yoo and J.-Q. Yu, J. Am. Chem. Soc., 2011, 133, 19598–19601 CrossRef CAS PubMed; (e) P. S. Thuy-Boun, G. Villa, D. Dang, P. F. Richardson, S. Su and J.-Q. Yu, J. Am. Chem. Soc., 2013, 135, 17508–17513 CrossRef CAS PubMed; (f) K. S. L. Chan, M. Wasa, L. Chu, B. N. Laforteza, M. Miura and J.-Q. Yu, Nat. Chem., 2014, 6, 146–150 CrossRef CAS PubMed; (g) K.-J. Xiao, D. W. Lin, M. Miura, R.-Y. Zhu, W. Gong, M. Wasa and J.-Q. Yu, J. Am. Chem. Soc., 2014, 136, 8138–8142 CrossRef CAS PubMed.
- (a) B. A. Steinhoff and S. S. Stahl, J. Am. Chem. Soc., 2006, 128, 4348–4355 CrossRef CAS PubMed; (b) K. L. Hull and M. S. Sanford, J. Am. Chem. Soc., 2007, 129, 11904–11905 CrossRef CAS PubMed; (c) K. L. Hull and M. S. Sanford, J. Am. Chem. Soc., 2009, 131, 9651–9653 CrossRef CAS PubMed; (d) A. Ishikawa, Y. Nakao, H. Sato and S. Sakaki, Dalton Trans., 2010, 39, 3279–3289 RSC; (e) B. P. Carrow and J. F. Hartwig, J. Am. Chem. Soc., 2011, 133, 2116–2119 CrossRef CAS PubMed.
- In the case of product 3f, attempts to improve reaction efficiency by the use of additional MPAA ligands (Boc-L-Thr-OH and Boc-L-Leu-OH) were unsuccessful and resulted in lower conversion.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5cc00410a |
| This journal is © The Royal Society of Chemistry 2015 |