Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
pH and light-controlled self-assembly of bistable [c2] daisy chain rotaxanes†
Adrian
Wolf
,
Emilie
Moulin
,
Juan-José
Cid
,
Antoine
Goujon
,
Guangyan
Du
,
Eric
Busseron
,
Gad
Fuks
and
Nicolas
Giuseppone
*
SAMS research group – University of Strasbourg – Institut Charles Sadron, CNRS, 23 rue du Loess, 67034 Strasbourg Cedex 2, BP 84087, France. E-mail: giuseppone@unistra.fr; Web: http://sams.ics-cnrs.unistra.fr/
First published on 22nd January 2015
Abstract
A logic gate based on a bistable [c2] daisy chain rotaxane decorated with lateral triarylamine units is described, giving rise to an INHIBIT logic function using proton concentration and light as inputs, and producing dual color change and supramolecular self-assembly as outputs.
Since the pioneering work of de Silva and collaborators,1 processing information by applying Boolean logic to chemical systems has been extensively developed.2 A large variety of synthetic molecules, even as simple as 8-methoxyquinoline, has been reported to achieve and integrate various kinds of Boolean operations.3 More advanced molecular machines, such as [2]pseudorotaxanes and [2]rotaxanes which present switching abilities upon chemical or physical stimulation, have also been designed with a particular focus on electronic devices with memory effects.4 Switchable molecular machines act by essence as logic gates and can reset a device to its initial state by taking advantage of their reversible bistability. Although various stimuli such as pH, metal ions, voltage, or even light have been used to activate these molecular logic units, examples combining light and pH inputs are limited.5 Outputs usually consist in an optical response such as UV/Vis absorption, fluorescence, phosphorescence, or circular dichroism as it provides an easy read-out of the molecular computation. However, examples in which an optical output is accompanied by the formation of a supramolecular self-assembly built on the molecular logic unit are scarce and mainly consist in the formation of physical gels.6
Recently we have discovered that triarylamine derivatives, when decorated with appropriate lateral amide groups, can self-assemble upon simple visible light exposure in the presence of chlorinated solvents thanks to a combination of various supramolecular interactions (hydrogen bond, π–π stacking, and van der Waals).7 Although the detailed mechanism of this unique supramolecular polymerization process is very complex, it can be summarized as follows: (i) oxidation upon light irradiation of a catalytic quantity of triarylamines to their radical cations associated with a chemical reduction of the chlorinated solvent; (ii) formation of a nucleus of a minimum number of triarylammonium radicals; (iii) stacking of neutral triarylamines onto the nucleus and subsequent growth of columnar primary fibrils; and (iv) lateral secondary aggregations of fibrils up to the formation of larger bundles of fibers.8 Interestingly, the formation of these self-assembled structures is accompanied by a strong increase in NIR absorption at around 790 nm corresponding to the formation and stabilization of the triarylammonium radical cations in charge transfer complexes. We have also shown that this nucleation/growth process leads to doped supramolecular polymers which display outstanding electric conduction properties with optical, magnetic, and electronic signatures of metallic materials.9 Finally, we have reported that these triarylamine units can be modified with various chemical units such as terpyridine, fullerene, or gallate moieties while retaining their general ability to self-assemble upon light stimulation in chlorinated solvents.10 However, during the course of our studies, we noticed the impact of the steric hindrance close to the amide function which is of importance to stabilize the self-assembled structures by intermolecular hydrogen bonds. Here we envision modulating the accessibility of this amide moiety by integrating a vicinal mechanical bond in order to further control the ability of the resulting switchable structure to self-assemble. For this purpose, we have designed bistable [c2] daisy chain rotaxanes decorated with triarylamine units as stoppers, and which can be actuated upon pH modulation.
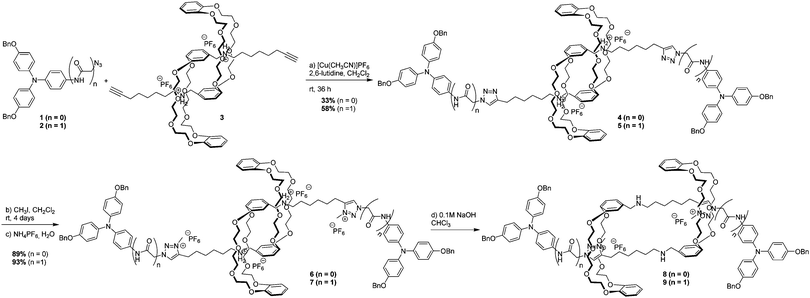
Two closely related [c2] daisy chain rotaxanes (6 without an amide group, and 7 incorporating an amide group) were synthesized from previously reported bisalkyne pseudorotaxane 311 and triarylamines 1 (see ESI†) and 27 using two key reactions: a copper-catalysed Huisgen [3+2] cycloaddition and a selective methylation of the triazole units (Scheme 1). The bistable nature of these rotaxanes is provided by the two binding sites on their axles: i.e. the secondary ammonium and the triazolium ions which have different affinity constants with the dibenzo-[24]crown-8 macrocycle.12 Whereas the secondary ammonium displays higher affinity for the macrocycle in extended 6 and 7, its deprotonation using a 0.1 M sodium hydroxide solution induces a shuttling of the crown ether to the triazolium site, as confirmed by 1H NMR of contracted 8 and 9.
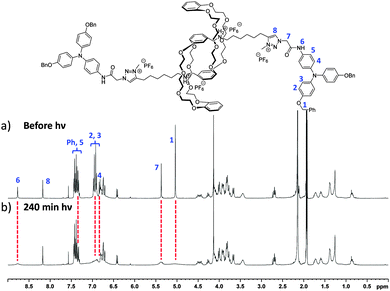
We then studied the behavior of triarylamine derivatives 4–9 upon visible light stimulation (in deuterated acetonitrile with 2.5% chloroform as electron acceptor, excepted for 4 and 5 which could be easily solubilized in pure chloroform). When the solutions were kept in the dark, all derivatives displayed the expected set of proton resonance signals, as illustrated by their 1H NMR spectra (Fig. 1a (7) and ESI†). However, after light irradiation using a 20 W lamp (power density: 0.07 W cm−2), the disappearance of the proton resonance signals corresponding to the self-assembly of the triarylamine core was observed for compounds 5 and 7 only (Fig. 1b (7) and Fig. S9 (5, ESI†)). This observation is in accordance with our previous studies showing the strong anisotropy of the triarylamine stacks and the paramagnetic contribution of the delocalized radical.7,10 For compounds 4, 6, and 8, which do not contain an amide group, the unchanged NMR signal upon light irradiation is in agreement with our previous studies mentioning the importance of the hydrogen bond interactions in the self-assembly of triarylamine derivatives. In the particular case of compound 9, the amide group necessary for self-assembly is strongly hindered by the spatial proximity of the macrocycle in this contracted form, thus precluding supramolecular polymerization. Importantly, treatment of the self-assembled structure made of 7 with NaOD led to the reappearance of NMR signals corresponding to the triarylamine core, thus demonstrating the reversibility of the self-assembly upon deprotonation of the rotaxane unit (Fig. S6 (ESI†)). Another signature of the self-assembly process was revealed by UV-Vis-NIR measurements, which are suitable to probe the stabilization of triarylammonium radicals in the self-assembly as a function of time upon light irradiation, owing to their characteristic broad absorption band centered at ∼760 nm (Fig. S11 (ESI†)). Whereas compounds 4, 6, 8 and 9 did not produce more than traces of radicals after 60 min of light irradiation, compounds 5 and 7 displayed a strong increase of the 760 nm absorption band, in correlation with their expected propensity to stack triarylamine moieties in charge transfer complexes but with different kinetics of aggregation (Fig. 2a).13 This experiment also confirmed the inability of compound 9 to stabilize radical cations, and thus to self-assemble, as determined by 1H NMR experiments. Similar conclusions were also made when compound 5 was deprotonated (i.e. contracted) using NaOH. In this case, NMR experiments suggest that the amide is also acting as a station for the macrocycle, thus increasing the steric hindrance nearby the triarylamine core and precluding hydrogen bond interactions necessary for the self-assembly process (Fig. S10 (ESI†)). Dynamic light scattering (DLS) experiments were then used to confirm the formation of supramolecular aggregates. In correlation with previous spectroscopic experiments, a single population of objects was observed for 5 and 7 after 60 min of light irradiation in a chloroform solution, displaying average hydrodynamic radii of 436 and 579 nm, respectively (Fig. 2b).
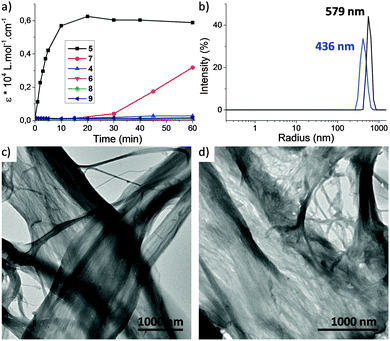 | ||
| Fig. 2 (a) Irradiation kinetic experiment plotted from NIR absorption at 760 nm for 0.1 mM solutions in chloroform of compounds 4–9. The corresponding UV-Vis-NIR spectra are reported in ESI;† (b) distributions of hydrodynamic radii observed for compound 7 (red) and 5 (black) after 60 min irradiation ([5] = [7] = 0.1 mM in CHCl3 at T = 25 °C and θ = 173°); (c and d) transmission electron micrographs of irradiated solutions of compounds 7 (c) and 5 (d) (prepared from 0.1 mM solutions in CHCl3). | ||
Transmission electron microscopy (TEM) was then used to image the structure of these self-assemblies (Fig. 2c (7) and d (5)). In both cases, the high contrast of the aggregates without staining agent revealed the presence of bundled fibrils of several microns length. Importantly, for compounds 4, 6, 8 and 9, DLS and TEM experiments confirmed the absence of self-assembled structures. Overall, the above results highlight that rotaxanes 5 and 7 execute an INHIBIT logic function with NIR absorption (green color of the solution) and fibrillar self-assembly as outputs. Indeed, changes in NIR absorption are only observed for their protonated forms and with light stimulation, thus defining light and base as inputs. The truth table corresponding to this INHIBIT logic gate can be defined as follows: (a) presence or absence of light stimulation as 1 and 0; (b) addition or not of base as 1 and 0; (c) presence or absence of NIR absorption at 760 nm (green color of the solution) as 1 and 0; (d) formation or not of a supramolecular self-assembly as 1 and 0, respectively (Table 1).
| Input 1 (white light) | Input 2 (base) | Output 1 (760 nm absorption) | Output 2 (self-assembly) |
|---|---|---|---|
| 0 | 0 | 0 | 0 |
| 0 | 1 | 0 | 0 |
| 1 | 0 | 1 | 1 |
| 1 | 1 | 0 | 0 |
In summary, we have reported the synthesis of bistable [c2] daisy chain rotaxanes decorated with amide substituted triarylamine units as stoppers. Using a combination of 1H NMR, UV-Vis absorption, DLS and TEM microscopy, we have demonstrated their light-triggered self-assembly into entangled micrometer-long supramolecular fibers thanks to the presence of light-induced triarylammonium radical cations. Furthermore, we have revealed their behavior as logic gate using a combination of pH and light as inputs. To the best of our knowledge, this work represents the first example of a molecular logic unit based on a [c2] daisy chain rotaxane, thus enlarging the scope of molecular machines applied to the field of molecular logic. Moreover, we expect that the formation of well-defined supramolecular self-assemblies could constitute an interesting new way to read out a logic device. Finally, these results enlarge the scope of chemical modifications that can be made on triarylamine cores while retaining their light-triggered self-assembly properties with potential interests in material science.
The research leading to these results has received funding from the European Research Council under the European Community's Seventh Framework Program (FP7/2007-2013, agreement no. 257099). We wish to thank the Centre National de la Recherche Scientifique, the COST action CM 1304, the international center for Frontier Research in Chemistry (fellowship to A.W.), the Laboratory of Excellence for Complex System Chemistry, the University of Strasbourg, and the Institut Universitaire de France for financial supports. This work was also supported by the Agence Nationale pour la Recherche (ANR-09-BLAN-034-02) and the French Ministry of Research (fellowships to J.J.C. and A.G.). Part of this work was performed within the framework of the IRTG “Soft Matter Science: Concepts for the Design of Functional Materials” (N.G. and A.W.).
Notes and references
- A. P. de Silva, H. Q. N. Gunaratne and C. P. Mccoy, Nature, 1993, 364, 42 CrossRef.
- (a) A. P. de Silva and S. Uchiyama, Nat. Nanotechnol., 2007, 2, 399 CrossRef PubMed; (b) K. Szaciłowski, Chem. Rev., 2008, 108, 3481 CrossRef PubMed; (c) J. Andréasson and U. Pischel, Chem. Soc. Rev., 2010, 39, 174 RSC; (d) E. Katz and V. Privman, Chem. Soc. Rev., 2010, 39, 1835 RSC; (e) G. Ashkenasy, Z. Dadon, S. Alesebi, N. Wagner and N. Ashkenasy, Isr. J. Chem., 2011, 51, 106 CrossRef.
- See for instance: (a) D. C. Magri, G. J. Brown, G. D. McClean and A. P. de Silva, J. Am. Chem. Soc., 2006, 128, 4950 CrossRef PubMed; (b) M. Ameli, M. Baroncini and A. Credi, Angew. Chem., Int. Ed., 2008, 47, 6240 CrossRef PubMed; (c) P. Ceroni, G. Bergamini and V. Balzani, Angew. Chem., Int. Ed., 2009, 48, 8516 CrossRef PubMed; (d) Z. Dadon, M. Samiappan, E. Y. Safranchik and G. Ashkenasy, Chem. – Eur. J., 2010, 16, 12096 CrossRef PubMed; (e) O. A. Bozdemir, R. Guliyev, O. Buyukcakir, S. Selcuk, S. Kolemen, G. Gulseren, T. Nalbantoglu, H. Boyaci and E. U. Akkaya, J. Am. Chem. Soc., 2010, 132, 8029 CrossRef PubMed; (f) J. Andréasson, U. Pischel, S. D. Straight, T. A. Moore, A. L. Moore and D. Gust, J. Am. Chem. Soc., 2011, 133, 11641 CrossRef PubMed; (g) D. S. Kim, V. M. Lynch, J. S. Park and J. L. Sessler, J. Am. Chem. Soc., 2013, 135, 14889 CrossRef PubMed; (h) J. Guo, J. Zhuang, F. Wang, K. R. Raghupathi and S. Thayumanavan, J. Am. Chem. Soc., 2014, 136, 2220 CrossRef PubMed; (i) K. S. Hettie, J. L. Klockow and T. E. Glass, J. Am. Chem. Soc., 2014, 136, 4877 CrossRef PubMed.
- (a) C. P. Carvalho and U. Pischel, Supramolecular Assemblies for Information Processing, in Molecular and Supramolecular Information Processing: From Molecular Switches to Logic Systems, ed. E. Katz, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2012 Search PubMed; (b) A. Credi, V. Balzani, S. J. Langford and J. F. Stoddart, J. Am. Chem. Soc., 1997, 119, 2679 CrossRef; (c) D.-H. Qu, Q.-C. Wang and H. Tian, Angew. Chem., Int. Ed., 2005, 44, 5296 CrossRef PubMed; (d) D. A. Leigh, M. Á. F. Morales, E. M. Pérez, J. K. Y. Wong, C. G. Saiz, A. M. Z. Slawin, A. J. Carmichael, D. M. Haddleton, A. M. Brouwer, W. J. Buma, G. W. H. Wurpel, S. León and F. Zerbetto, Angew. Chem., Int. Ed., 2005, 44, 3062 CrossRef PubMed; (e) J. E. Green, J. W. Choi, A. Boukai, Y. Bunimovich, E. Johnston-Halperin, E. DeIonno, Y. Luo, B. A. Sheriff, K. Xu, Y. S. Shin, H.-R. Tseng, J. F. Stoddart and J. R. Heath, Nature, 2007, 445, 414 CrossRef PubMed; (f) C. Jia, H. Li, J. Jiang, J. Wang, H. Chen, D. Cao, J. F. Stoddart and X. Guo, Adv. Mater., 2013, 25, 6752 CrossRef PubMed; (g) J. Cao, X. Ma, M. Min, T. Cao, S. Wu and H. Tian, Chem. Commun., 2014, 50, 3224 RSC; (h) F. Lohmann, J. Weigandt, J. Valero and M. Famulok, Angew. Chem., Int. Ed., 2014, 53, 10372 CrossRef PubMed; (i) U. Pischel, Chimia, 2014, 68, 505 CrossRef PubMed.
- (a) F. M. Raymo, S. Giordani, A. J. P. White and D. J. Williams, J. Org. Chem., 2003, 68, 4158 CrossRef PubMed; (b) L. Zhao, S. Wang, Y. Wu, Q. Hou, Y. Wang and S. Jiang, J. Phys. Chem. C, 2007, 111, 18387 CrossRef; (c) S. Angelos, Y.-W. Yang, N. M. Khashab, J. F. Stoddart and J. I. Zink, J. Am. Chem. Soc., 2009, 131, 11344 CrossRef PubMed; (d) H. Komatsu, S. Matsumoto, S. Tamaru, K. Kaneko, M. Ikeda and I. Hamachi, J. Am. Chem. Soc., 2009, 131, 5580 CrossRef PubMed; (e) M. Semeraro and A. Credi, J. Phys. Chem. C, 2010, 114, 3209 CrossRef.
- (a) J. W. Chung, S.-J. Yoon, S.-J. Lim, B.-K. An and S. Y. Park, Angew. Chem., Int. Ed., 2009, 48, 7030 CrossRef PubMed; (b) Z. Qi, P. M. de Molina, W. Jiang, Q. Wang, K. Nowosinski, A. Schulz, M. Gradzielski and C. A. Schalley, Chem. Sci., 2012, 3, 2073 RSC; (c) K. Fan, J. Yang, X. Wang and J. Song, Soft Matter, 2014, 10, 8370 RSC.
- E. Moulin, F. Niess, M. Maaloum, E. Buhler, I. Nyrkova and N. Giuseppone, Angew. Chem., Int. Ed., 2010, 49, 6974 CrossRef PubMed.
- I. Nyrkova, E. Moulin, J. J. Armao IV, M. Maaloum, B. Heinrich, M. Rawiso, F. Niess, J.-J. Cid, N. Jouault, E. Buhler, A. Semenov and N. Giuseppone, ACS Nano, 2014, 8, 10111 CrossRef PubMed.
- (a) V. Faramarzi, F. Niess, E. Moulin, M. Maaloum, J.-F. Dayen, J.-B. Beaufrand, S. Zanettini, B. Doudin and N. Giuseppone, Nat. Chem., 2012, 4, 485 CrossRef PubMed; (b) J. J. Armao IV, M. Maaloum, T. Ellis, G. Fuks, M. Rawiso, E. Moulin and N. Giuseppone, J. Am. Chem. Soc., 2014, 136, 11382 CrossRef PubMed; (c) A. Akande, S. Bhattacharya, T. Cathcart and S. Sanvito, J. Chem. Phys., 2014, 074301 CrossRef PubMed; (d) S. Bhattacharya, A. Akande and S. Sanvito, Chem. Commun., 2014, 50, 6626 RSC; (e) E. Moulin, J.-J. Cid and N. Giuseppone, Adv. Mater., 2013, 25, 477 CrossRef PubMed.
- (a) E. Moulin, F. Niess, G. Fuks, N. Jouault, E. Buhler and N. Giuseppone, Nanoscale, 2012, 4, 6748 RSC; (b) Y. Domoto, E. Busseron, M. Maaloum, E. Moulin and N. Giuseppone, Chem. – Eur. J., 2015, 1938 CrossRef CAS PubMed.
- (a) F. Coutrot, C. Romuald and E. Busseron, Org. Lett., 2008, 10, 3741 CrossRef PubMed; (b) G. Du, E. Moulin, N. Jouault, E. Buhler and N. Giuseppone, Angew. Chem., Int. Ed., 2012, 51, 12504 CrossRef PubMed.
- (a) F. Coutrot and E. Busseron, Chem. – Eur. J., 2008, 14, 4784 CrossRef PubMed; (b) C. Romuald, A. Ardá, C. Clavel, J. Jiménez-Barbero and F. Coutrot, Chem. Sci., 2012, 3, 1851 RSC; (c) C. Romuald, E. Busseron and F. Coutrot, J. Org. Chem., 2010, 75, 6516 CrossRef PubMed; (d) Y. Jiang, J.-B. Guo and C.-F. Chen, Org. Lett., 2010, 12, 4248 CrossRef PubMed.
- The kinetics of aggregation of triarylamine amides derivatives is dependent on their ability to form and stabilize radical cations in stacked charge transfer complexes. Whereas the self-assembly kinetic of compound 5 is very similar to the one already reported for related derivatives in ref. 7, 8 or 10, a significantly slower kinetic of aggregation is observed for compound 7. One can argue that the presence of the triazolium decreases the nucleation rate of the charge transfer complexes due to electrostatic repulsions between triazolium moieties.
Footnote |
| † Electronic supplementary information (ESI) available: General procedures, synthetic protocols, 1H and 13C NMR spectra of compounds 4–9, UV spectra of compounds 4–9. See DOI: 10.1039/c4cc10331a |
| This journal is © The Royal Society of Chemistry 2015 |