Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Selective fluorogenic imaging of hepatocellular H2S by a galactosyl azidonaphthalimide probe†
De-Tai
Shi
a,
Dan
Zhou
ab,
Yi
Zang
b,
Jia
Li
*b,
Guo-Rong
Chen
a,
Tony D.
James
c,
Xiao-Peng
He
*a and
He
Tian
a
aKey Laboratory for Advanced Materials & Institute of Fine Chemicals, East China University of Science and Technology, 130 Meilong Rd., Shanghai 200237, P. R. China. E-mail: xphe@ecust.edu.cn
bNational Center for Drug Screening, State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, 189 Guo Shoujing Rd., Shanghai 201203, P. R. China. E-mail: jli@simm.ac.cn
cDepartment of Chemistry, University of Bath, Bath, BA2 7AY, UK
First published on 21st January 2015
Abstract
We have developed a galactosyl azidonaphthalimide probe for the selective fluorogenic imaging of hepatocellular H2S, an important gaseous transmitter produced in the liver.
Despite its toxicity, hydrogen sulfide (H2S) has been identified as an important gaseous transmitter in many cellular signalling pathways.1 Cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE) are the two major enzymes responsible for the production of H2S with L-(homo)cystein as the substrate. Because of the tissue-specific distribution of these enzymes, H2S is predominantly generated in the liver.2 However, this metabolic organ is vulnerable to damage from the aberrant expression of redox-active species including H2S, leading to hepatic diseases such as liver cirrhosis.3 As a result, selective detection of hepatocellular H2S represents an important goal to aid not only cell biology research but also disease diagnosis.
Conventional techniques for H2S detection depend on chromatographic and electrochemical sensors, which are not applicable for live cell imaging.4 To address these issues, numerous fluorescence small-molecule probes for H2S have been developed over recent years. The basis for the fluorogenic response (or fluorescence “OFF–ON”) of the probes relies on H2S-mediated reduction of azides,5,6 copper sulfide precipitation7,8 and nucleophilic addition strategies.9–11 All these probe prototypes address sensitive and selective detection of the species, even in live cells and in vivo.12–14 Nevertheless, although elegant H2S probes with cellular trapping,15 lysosome16 and mitochondria targeting17 abilities have been developed, fluorogenic probes that address hepatocyte-selective imaging of the species are rare.
Recently, we18–21 and others22–26 determined that the introduction of a glycosyl moiety (as a targeting agent) to a fluorescence probe can greatly enhance its selective internalisation by cells derived from a certain tissue. This could be ascribed to the selective, sugar-receptor-mediated endocytosis of the glyco-probes by the cells. Here we show that a galatosyl azidonaphthalimide probe has the ability to image H2S selectively in hepatocytes among other cells, due to galactoside receptor-promoted endocytosis.
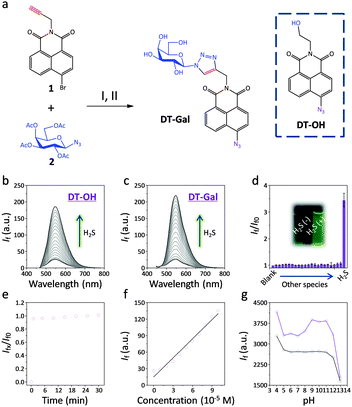
The desired DT-Gal was synthesised by a click-coupling of alkynyl naphthalimide 1 with azido galactoside 2, followed by a sequential azidation and deacylation (Fig. 1a). The presence of the azide weakens the fluorescence of naphthalimide by an ICT process, and a subsequent reduction by H2S produces aminonaphthalimide, enhancing the fluorescence.14DT-OH which is a known H2S probe that lacks the galactosyl targeting agent was used as a control.27
With these probes in hand, we tested their fluorescence response to H2S in an aqueous solution (PBS, pH 7.4). The presence of increasing H2S caused a gradual fluorescence increase of both DT-OH (Fig. 1b) and DT-Gal (Fig. 1c) with the latter being more sensitive. This might be a result of the better water solubility of the latter glyco-probe. Among a range of anions (Fig. 1d) and amino acids (Fig. S1, ESI†), both probes showed good selectivity. Mass spectroscopic analysis indicated that the aminonaphthalimide derivative of DT-Gal was produced upon reaction with H2S (Fig. S2, ESI†).
A kinetic investigation determined that DT-Gal showed rapid fluorogenic response to H2S (Fig. 1e), and good linearity was observed by plotting the fluorescence intensity of the probe as a function of increasing H2S (Fig. 1f). The limit of detection of DT-Gal for H2S was determined to be 0.78 μM (3σb/k). Additionally, we observed that the probe functioned well over a wide pH range (4–12, Fig. 1g), suggesting its applicability for cellular analysis.
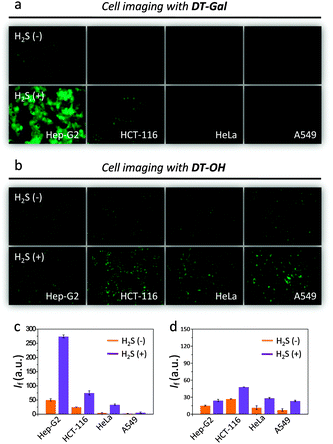
Subsequently, we tested the ability of the probes to image H2S in live cells. We used the human hepatoma cell line (Hep-G2) with over-expressed asialoglycoprotein receptor (ASGPr) that recognises galactosides18–21 as well as cancer cells without the receptor expression such as human colon cancer HCT116, cervix cancer HeLa and lung cancer A549.18 We observed that, the addition of DT-Gal to the cell lines pre-treated with H2S led to fluorescence enhancement for Hep-G2, but only a slight fluorescence increase for other cells (Fig. 2a and c). In sharp contrast, co-incubation of DT-OH with the cells led to slight, unselective fluorescence enhancement (Fig. 2b and d). These results suggest that the presence of the galactosyl warhead facilitates the selective internalisation of the H2S probe probably by ASGPr-mediated endocytosis.18–21 Notably, the assumed endocytosis led to much stronger fluorescence generation of DT-Gal than the DT-OH without a targeting group.
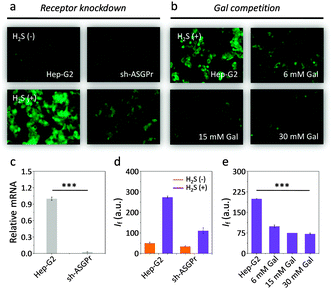
To corroborate that the imaging was a result of selective ASGPr–galactose interaction, we used a Hep-G2 cell line with a reduced ASGPr expression level (sh-ASGPr)21 to incubate with DT-Gal. We observed that addition of the galactosyl probe to sh-ASGPr resulted in a much weaker fluorescence compared to Hep-G2 (Fig. 3a and d). This correlates with the distinct ASGPr expression level of the two cell lines (Fig. 3c). Moreover, pre-incubation of increasing free galactose with Hep-G2 inhibited the fluorescence enhancement of DT-Gal in a concentration-dependent manner (Fig. 3b and e). These data support the assumption that the strong and selective fluorogenic signal produced by DT-Gal in raw Hep-G2 cells is facilitated by ASGPr-mediated endocytosis.
We also tested the kinetics of DT-Gal for H2S imaging in the hepatocellular cell line. We observed a time-dependent fluorescence enhancement of the imaging experiments and equilibrium was achieved in about 30 min (Fig. 4a and b). Note that this cellular kinetics is slower than that in solution probably because of the complexity of the cellular environment. Interestingly, DT-Gal (Fig. 4c) was much less toxic towards Hep-G2 as well as a human kidney cell line (HEK293) than DT-OH (Fig. 4d). This suggests that, in addition to its targeting ability, the presence of the galactosyl moiety might also mitigate the intrinsic cytotoxicity of naphthalimide-based compounds.28
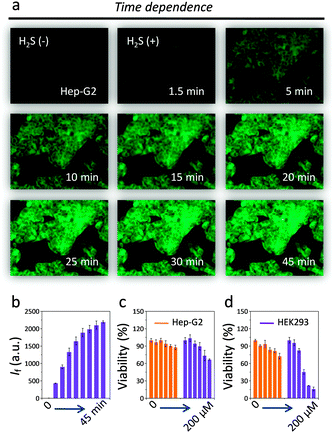 | ||
| Fig. 4 Cell imaging (a) and fluorescence quantification (b) of DT-Gal for Hep-G2 with increasing incubation time. Cytotoxicity of (c) DT-Gal (0–200 μM) and (d) DT-OH (0–200 μM) for Hep-G2 and HEK293. | ||
We note that a similar azidonaphthalimide probe for H2S has been previously reported.29 However, unlike our DT-Gal probe, that probe does not contain a cellular targeting unit, which is vital for target-specific imaging in vivo. Since azidonaphthalimide can be reduced to the fluorescent amino derivative within cells,25 we carried out a time-dependent imaging assay with Hep-G2 and just DT-Gal (Fig. S3, ESI†). While the fluorescence increased slightly, the co-existence of H2S results in significantly enhanced fluorescence. This clearly demonstrates that the fluorescence enhancements observed here are due to H2S recognition within the cells.
In conclusion, a galactosyl azidonaphthalimide based fluorogenic probe for hepatocellular-selective imaging of H2S was developed, which sets a basis for the target-specific imaging of H2S in the liver, the main organ that produces this gaseous transmitter.
This research is supported by the 973 project (2013CB733700), the National Science Fund for Distinguished Young Scholars (81125023), the National Natural Science Foundation of China (21176076, 21202045 and 91213303), the Program of Shanghai Subject Chief Scientist (13XD1404300), the Key Project of Shanghai Science and Technology Commission (13NM1400900) and the Fundamental Research Funds for Central Universities. The Catalysis And Sensing for our Environment (CASE) network is thanked for research exchange opportunities.
Notes and references
- P. Kamoun, Amino Acids, 2004, 26, 243 CrossRef CAS PubMed.
- J. Yan, F. Teng, W. Chen, Y. Ji and Z. Gu, Exp. Ther. Med., 2012, 4, 832 CAS.
- S. Fiorucci, E. Antonelli, A. Mencarelli, S. Orlandi, B. Renga, G. Rizzo, E. Distrutti, B. Shah and A. Morelli, Hepatology, 2005, 42, 539 CrossRef CAS PubMed.
- A. Tangerman, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2009, 28, 3366 CrossRef PubMed.
- A. R. Lippert, E. J. New and C. J. Chang, J. Am. Chem. Soc., 2011, 133, 10078 CrossRef CAS PubMed.
- H. Peng, Y. Cheng, C. Dai, A. L. King, B. L. Predmore, D. J. Lefer and B. Wang, Angew. Chem., Int. Ed., 2011, 50, 9672 CrossRef CAS PubMed.
- K. Sasakura, K. Hanaoka, N. Shibuya, Y. Mikami, Y. Kimura, T. Komatsu, T. Ueno, T. Terai, H. Kimura and T. Nagano, J. Am. Chem. Soc., 2011, 133, 18003 CrossRef CAS PubMed.
- F. Hou, L. Huang, P. Xi, J. Cheng, X. Zhao, G. Xie, Y. Shi, F. Cheng, X. Yao, D. Bai and Z. Zeng, Inorg. Chem., 2012, 51, 2454 CrossRef CAS PubMed.
- Z. Xu, L. Xu, J. Zhou, Y. Xu, W. Zhu and X. Qian, Chem. Commun., 2012, 48, 10871 RSC.
- X. Cao, W. Lin, K. Zheng and L. He, Chem. Commun., 2012, 48, 10529 RSC.
- Y. Chen, C. Zhu, Z. Yang, J. Chen, Y. He, Y. Jiao, W. He, L. Qiu, J. Cen and Z. Guo, Angew. Chem., Int. Ed., 2013, 52, 1688 CrossRef CAS PubMed.
- W. Xuan, C. Sheng, Y. Cao, W. He and W. Wang, Angew. Chem., Int. Ed., 2012, 51, 2282 CrossRef CAS PubMed.
- V. S. Lin and C. J. Chang, Curr. Opin. Chem. Biol., 2012, 16, 595 CrossRef CAS PubMed.
- F. Yu, X. Han and L. Chen, Chem. Commun., 2014, 50, 12234 RSC.
- V. S. Lin, A. R. Lippert and C. J. Chang, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 7131 CrossRef CAS PubMed.
- T. Liu, Z. Xu, D. R. Spring and J. Cui, Org. Lett., 2013, 15, 2301 Search PubMed.
- S. K. Bae, C. H. Heo, D. J. Choi, D. Sen, E. H. Joe, B. R. Cho and H. M. Kim, J. Am. Chem. Soc., 2013, 135, 9915 CrossRef CAS PubMed.
- K.-B. Li, Y. Zang, H. Wang, J. Li, G.-R. Chen, T. D. James, X.-P. He and H. Tian, Chem. Commun., 2014, 50, 11735 RSC.
- X.-P. He, R.-H. Li, S. Maisonneuve, Y. Ruan, G.-R. Chen and J. Xie, Chem. Commun., 2014, 50, 14141 RSC.
- H.-L. Zhang, Y. Zang, J. Xie, J. Li, G.-R. Chen, X.-P. He and H. Tian, Sci. Rep., 2014, 4, 5513 Search PubMed.
- H.-L. Zhang, X.-L. Wei, Y. Zang, J.-Y. Cao, S. Liu, X.-P. He, Q. Chen, Y.-T. Long, J. Li, G.-R. Chen and K. Chen, Adv. Mater., 2013, 25, 4097 CrossRef CAS PubMed.
- K. EI-Boubbou, D. C. Zhu, C. Vasileiou, B. Borhan, D. Prosperi, W. Li and X. Huang, J. Am. Chem. Soc., 2010, 132, 4490 CrossRef PubMed.
- M. H. Lee, J. H. Han, P.-S. Kwon, S. Bhuniya, J. Y. Kim, J. L. Sessler, C. Kang and J. S. Kim, J. Am. Chem. Soc., 2012, 134, 1316 CrossRef CAS PubMed.
- K. Y. Zhang, K. K.-S. Tso, M.-W. Louie, H.-W. Liu and K. K.-W. Lo, Organometallics, 2013, 32, 5098 CrossRef CAS.
- M. Sawa, T.-L. Hsu, T. Itoh, M. Sugiyama, S. R. Hanson, P. K. Vogt and C.-H. Wong, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 12371 CrossRef CAS PubMed.
- D. Wu, S. Cheung, R. Daly, H. Burke, E. M. Scanlan and D. F. O’Shea, Eur. J. Org. Chem., 2014, 6841 CrossRef CAS.
- C. Yu, X. Li, F. Zeng, F. Zheng and S. Wu, Chem. Commun., 2013, 49, 403 RSC.
- X. Li, Y. Lin, Q. Wang, Y. Yuan, H. Zhang and X. Qian, Eur. J. Med. Chem., 2011, 46, 1274 CrossRef CAS PubMed.
- L. A. Montoya and M. D. Pluth, Chem. Commun., 2012, 48, 4767 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section and additional figures. See DOI: 10.1039/c4cc09771h |
| This journal is © The Royal Society of Chemistry 2015 |