Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Infrared laser triggered release of bioactive compounds from single hard shell microcapsules†
Tobias
Vöpel
a,
Rebecca
Scholz
bc,
Luca
Davico
b,
Magdalena
Groß
a,
Steffen
Büning
a,
Sabine
Kareth
bc,
Eckhard
Weidner
bc and
Simon
Ebbinghaus
*a
aDepartment of Physical Chemistry II, Ruhr-University Bochum, Universitätsstr. 150, 44780 Bochum, Germany. E-mail: Simon.Ebbinghaus@rub.de; Tel: +49 234-3225533
bChair of Process Technology, Ruhr-University Bochum, Germany
cFraunhofer Institute UMSICHT, Oberhausen, Germany
First published on 9th February 2015
Abstract
Micro composites are commonly characterized in bulk. Here we study the temperature triggered release of a bioactive compound from single isolated microcapsules. We monitor the release process in real-time using a novel thermal microscopy method combining laser-induced heating and fluorescence imaging.
Microencapsulation of functional compounds (for example flavors, proteins or vitamins) can be of great value for the use in food, cosmetic or pharmaceutical industry.1–6 Encapsulation is a way to increase shelf life, improve stability and control the release of the encapsulated active compounds.7
For the analysis and characterization of microcapsule products, techniques such as Differential Scanning Calorimetry,8 Nuclear Magnetic Resonance,9 thermogravimetric analysis10 or viscosity-measurements are applied. Furthermore, bright field or electron microscopy are used to determine particle size, shape and surface characteristics.11 These techniques report sample averaged properties, without accounting for heterogeneity of the samples. To characterize the melting of temperature sensitive, single microcapsules with high spatio-temporal resolution we implement a new tool to trigger and visualize the release of active compounds. The technique combines infrared laser heating and high speed microscopy12 (Scheme 1). In comparison to other thermal microscopy methods which evenly heat the whole sample13 this method heats microcapsules in the focal spot of the laser only. To demonstrate the method, we produced a model system by entrapping a bioactive compound in a temperature sensitive, hard shell microcapsule.
There are various encapsulation techniques available to entrap actives like spray-drying, freeze-drying, rapid expansion of supercritical solutions (RESS), coacervation, particle from gas saturated solutions (PGSS), biopolymerization and emulsification.20,21 Drawbacks, however, are increased costs and the additional complexity of handling the encapsulation process. For the preparation of our model system we have chosen the hot melt dispersion technique,14,15 as it is cost effective, easily executable and can be performed in lab scale as well as in industrial scale. The hard fat Witepsol W31, which has a melting temperature close to the human body temperature, was used as the shell material. As core material enhanced Yellow Fluorescent Protein (eYFP) was selected. eYFP is derived from the Green Fluorescent Protein (GFP).16 eYFP and other fluorescent proteins are widely used as reporters in cells or animals.18,19 It has a molecular weight of 27 kDa and is mainly composed of β-sheets arranged in a barrel like shape. The barrel embraces the chromophore and shields it from the environment.17 The melting temperature of the protein is 78 °C, similar to the melting temperature of GFP.16 The protein emits fluorescent light at a maximum of 527 nm when excited at 514 nm. Oxidative stress, denaturants or degradation can damage the fluorophore and lead to a decay of its fluorescence. This allows the implementation of its fluorescence as a marker for native protein encapsulation. In this work we show that the technique can be used to determine the melting temperature of single microcapsules and to image the melting process and compound release.
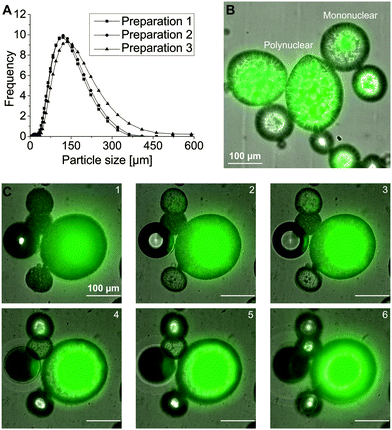
We prepared the capsules using the hot melt dispersion technique. This technique utilizes a water in oil in water (W/O/W) emulsion which was rapidly cooled down to solidify the Witepsol W 31 shell material to form stable capsules. The production process led to a reproducible, narrow particle size distribution with an average d50 of 130 ± 14 μm and a span of 1.4 ± 0.1 (Fig. 1A). The used preparation parameters resulted in different capsule types which were categorized as mono-or polynuclear in morphology (Fig. 1B).22 A representative, tomographic view of a mononuclear core–shell capsule is shown in Fig. 1C. The capsule was 185 μm in diameter with a core diameter of 110 μm. Localization of the fluorescence signal in the core region showed encapsulation of eYFP. Assuming a spherical shape for both the capsule and the core, a core![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) capsule volume ratio of 1
capsule volume ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 was calculated.
5 was calculated.
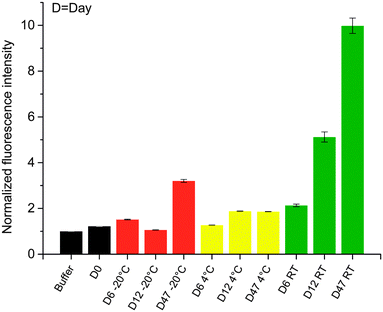
To demonstrate the stability of the micro release system and its ability to entrap eYFP in its native state at specified storage conditions, 25 mg of the capsules were added to Dulbecco's phosphate-buffered saline (DPBS) and stored at −20 °C, 4 °C and room temperature (RT) over a time period of 7 weeks, respectively. At different time points, the fluorescence intensity of the buffers was determined to detect leaked proteins (Fig. 2). The sample stored at RT showed significant leakage of the protein after 12 days. However, the shelf life of the micro release system can be increased by storing it at cooled (4 °C) or frozen (−20 °C) conditions.
We then used the thermal microscopy technique to study the temperature triggered release of eYFP from individual capsules. The capsules were melted in DPBS by infrared laser heating at 2200 nm (see ESI†) with the laser focused to heat up a volume of 0.20 mm3. The melting process of the microcapsule was then imaged by fluorescence microscopy. The laser power was calibrated to heat the sample by using the temperature dependent fluorescence of Rhodamine B (see ESI†). Typical heating profiles are shown in Fig. S1 (ESI†). The dimensions of the heating spot were selected larger in size compared to the average microcapsule diameter to heat every microcapsule uniformly. Thereby, local temperature gradients across the capsule that could affect the release properties were avoided. However, small laser foci are desirable to selectively heat preselected single microcapsules in the sample (Fig. S1, ESI†). This is a great advantage in comparison to methods where the entire sample chamber is heated such as hot-stage microscopy.13 Using such methods, all microcapsules loaded to the sample chamber melt and release the bioactive during a single temperature scan. However, as high resolution microscopy is required to monitor the release, only a very few microcapsules can be analyzed and subsequently an exchange of the entire sample is required.
The technique can be operated in two different modes. A fast temperature increase to instantaneously trigger the release of the capsule or successive temperature increases to precisely determine its melting temperature. In the first mode the release is triggered by modulating the laser power output waveform to achieve a single increase in temperature from RT up to the melting temperature (Fig. 3A, red curve). The time course of melting a mononuclear capsule is shown in Video S1 (ESI†). A region of interest (ROI) analysis of the Video S1 (ESI†) is shown in Fig. 3. ROI's at cardinal and ordinal positions around the capsule were defined to track the fluorescence intensity during the experiment. After 14 seconds which corresponds to a temperature increase of 10 °C above RT the shell breaks at a single spot in the upper left part of the capsule and the liquid core, which contains eYFP, leaked into the surrounding solution. This lead to a major increase in fluorescence of the surrounding ROI's N, W and NW. The fluorescence dissipates over time returning to its initial value (Fig. 3). The rapid heating mode allows to trigger and monitor the release of an entrapped bioactive from single microcapsules within a few seconds. This additionally reduces the time to characterize the release properties in comparison to hot-stage microscopy which usually operates with heating rates up to 10 °C per minute.13
Secondly, the thermal microscopy technique can be used to slowly increase the temperature to precisely determine the melting temperature of the capsules shell. The laser power output waveform was modulated to create 25 individual heating steps each of 1 °C with 10 s of constant laser power between each step, thus heating the sample up to 50 °C (Fig. 4). In the initial heating phase the detected fluorescence intensity around the particle was unchanged showing that the integrity of the shell is not compromised. At 35 °C a slight increase in the fluorescence intensity of ROI N due to a displacement of the capsule was detected. At 36 °C (Fig. 4) the capsule breaks at a single spot and the liquid core leaked into the surrounding buffer (see Video S2, ESI† at time 01![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 min). This was accompanied by an increase in the fluorescence intensity of the surrounding buffer, in particular for ROI's E, S and SE (Fig. 4A). The regions W and SW are on the far side of the release and showed only a minor increase in the fluorescence intensity. The eYFP protein that diffused into the buffer led to an increase in the normalized fluorescence intensity which returned to its initial value. After the laser is turned off, the sample cooled down to RT which resulted in a rapid solidification of the molten fat. The particle thereby formed amorphous structures and lost its spherical shape (Fig. 4B). The melting temperature of 36 °C at which the core was released into the buffer corresponds well with the melting temperature of 35–37 °C reported for the pure fat Witepsol W31.
40 min). This was accompanied by an increase in the fluorescence intensity of the surrounding buffer, in particular for ROI's E, S and SE (Fig. 4A). The regions W and SW are on the far side of the release and showed only a minor increase in the fluorescence intensity. The eYFP protein that diffused into the buffer led to an increase in the normalized fluorescence intensity which returned to its initial value. After the laser is turned off, the sample cooled down to RT which resulted in a rapid solidification of the molten fat. The particle thereby formed amorphous structures and lost its spherical shape (Fig. 4B). The melting temperature of 36 °C at which the core was released into the buffer corresponds well with the melting temperature of 35–37 °C reported for the pure fat Witepsol W31.
The model release system presented here was prepared using the hot melt dispersion technique. The capsules are easily prepared and their properties can be tuned by altering parameters like stirring speed or the selection of the shell material. Our results emphasize that hot melt dispersion is an easily executable and scalable emulsification technique that can be used to prepare hard shell microcapsules for the temperature controlled, instantaneous release of bioactive compounds like proteins or peptides for therapeutic delivery. Furthermore, it can be extended to encapsulate active food ingredients like probiotics. We demonstrate a novel thermal microscopy method for single capsule characterization of temperature triggered release systems. The technique was used to trigger and monitor the release of an encapsulated biological active from a model micro release system. The integrated heating laser can be tuned to rapidly trigger the release of the liquid core or to probe the thermal melting curve with centigrade precision by a stepwise temperature increase. The release and the melting temperature of individual microcapsules can be analyzed and correlated to bulk properties studying size-dependent effects. Future development of the technique will allow to use the described method in a high throughput fashion.
We acknowledge funding from the Ministry of Innovation, Science and Research of the State of North Rhine-Westphalia (Rückkehrerprogramm), the Cluster of Excellence RESOLV (EXC 1069) funded by the German Research Foundation (DFG) and the People programme (Marie Curie Actions) of the European Union's Seventh Framework Programme FP7/2007–2013/ under REA grant agreement no. 316959 (DoHip project, “Training Program for the Design of Resource and Energy Efficient Products by High Pressure Processes”).
Notes and references
- S. D. Braun and N. F. Olson, J. Microencapsulation, 1986, 3, 115–126 CrossRef CAS PubMed.
- K. G. H. Desai and H. J. Park, Drying Technol., 2005, 23, 1361–1394 CrossRef CAS PubMed.
- M. H. Lee, S. G. Oh, S. K. Moon and S. Y. Bae, J. Colloid Interface Sci., 2001, 240, 83–89 CrossRef CAS PubMed.
- N. Mongenot, S. Charrier and P. Chalier, J. Agric. Food Chem., 2000, 48, 861–867 CrossRef CAS PubMed.
- A. SilvaCunha, J. L. Grossiord, F. Puisieux and M. Seiller, Int. J. Pharm., 1997, 158, 79–89 CrossRef CAS.
- N. Wilson and N. P. Shah, ASEAN Food J., 2007, 14, 1–14 Search PubMed.
- J. D. Oxley, Agro Food Ind. Hi-Tech, 2012, 23, 60–63 Search PubMed.
- L. G. Hanu, P. Alessi, A. Kilzer and S. Kareth, J. Supercrit. Fluids, 2012, 66, 274–281 CrossRef CAS PubMed.
- S. Leick, M. Kott, P. Degen, S. Henning, T. Pasler, D. Suter and H. Rehage, Phys. Chem. Chem. Phys., 2011, 13, 2765–2773 RSC.
- H. H. Horowitz and G. Metzger, Anal. Chem., 1963, 35, 1464–1468 CrossRef CAS.
- S. Jaspart, G. Piel, L. Delattre and B. Evrard, Expert Opin. Drug Delivery, 2005, 2, 75–87 CrossRef CAS PubMed.
- S. Ebbinghaus, A. Dhar, J. D. McDonald and M. Gruebele, Nat. Methods, 2010, 7, 319–323 CrossRef CAS PubMed.
- I. M. Vitez, A. W. Newman, M. Davidovich and C. Kiesnowski, Thermochim. Acta, 1998, 324, 187–196 CrossRef CAS.
- R. Bodmeier, J. Wang and H. Bhagwatwar, J. Microencapsulation, 1992, 9, 89–98 CrossRef CAS PubMed.
- R. Bodmeier, J. Wang and H. Bhagwatwar, J. Microencapsulation, 1992, 9, 99–107 CrossRef CAS PubMed.
- R. Y. Tsien, Annu. Rev. Biochem., 1998, 67, 509–544 CrossRef CAS PubMed.
- A. Rekas, J. R. Alattia, T. Nagai, A. Miyawaki and M. Ikura, J. Biol. Chem., 2002, 277, 50573–50578 CrossRef CAS PubMed.
- A. Miyawaki, Microscopy, 2013, 62, 63–68 CrossRef CAS PubMed.
- R. Yuste, Nat. Methods, 2005, 2, 902–904 CrossRef CAS PubMed.
- Z. Knez and E. Weidner, Curr. Opin. Solid State Mater. Sci., 2003, 7, 353–361 CrossRef CAS PubMed.
- N. J. Zuidam, Encapsulation Technologies for Active Food Ingredients and Food Processing, Springer Science + Business Media, New York, NY, 2010 Search PubMed.
- S. K. Ghosh, Functional Coatings and Microencapsulation, Wiley-VCH Verlag GmbH & Co. KGaA, 2006 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Materials and methods and supplemental Videos S1–S3. See DOI: 10.1039/c4cc09745a |
| This journal is © The Royal Society of Chemistry 2015 |