Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Reversible Janus particle assembly via responsive host–guest interactions†
Ying
Zhou
ab,
Dongsheng
Wang
a,
Shilin
Huang
a,
Günter
Auernhammer
a,
Yujian
He
b,
Hans-Jürgen
Butt
a and
Si
Wu
*a
aMax Planck Institute for Polymer Research, Ackermannweg 10, 55128 Mainz, Germany. E-mail: wusi@mpip-mainz.mpg.de
bCollege of Chemistry and Chemical Engineering, University of Chinese Academy of Sciences, 19A YuQuan Road, Beijing 100049, China
First published on 6th January 2015
Abstract
Reversible assembly of Janus particles was manipulated by host–guest interaction of β-cyclodextrin (β-CD) and azobenzene. One side of every Janus particle was modified with β-CD. Superstructures of Janus particles were formed by adding azobenzene-containing polymers to the dispersion of Janus particles. The superstructures were reversibly disassembled by adding α-CD or light irradiation.
Janus particles exhibit distinct physicochemical properties on their opposite sides. A few years ago, de Gennes highlighted the novelty of Janus particles in his Nobel lecture.1 One novel feature of Janus particles is their self-assembly. The study of Janus particle assembly provides more general fundamental understanding on self-assembly.2 Moreover, Janus particles can assemble into unique superstructures that could not be obtained by the assembly of homogeneous particles.2
To date, most superstructures prepared by Janus particle assembly are static;2,3i.e., the superstructures are not stimuli-responsive. To construct responsive superstructures of Janus particles, the bonding between Janus particles should be switchable.4 Superstructures of Janus particles assembled by DNA linkers and wetting-induced attraction can be switched by changing the temperatures of the systems in water and water/2,6-lutidine mixtures, respectively.4a,b These results nicely demonstrated the feasibility of using Janus particles for stimuli-responsive materials. To push Janus particle assembly one step further toward stimuli-responsive materials, some challenges remain. For example, for many applications of stimuli-responsive materials, water and water/2,6-lutidine mixtures may not be the preferred environments, and temperature may not be the desirable stimulus. To make assembly of Janus particles a general method for fabricating stimuli-responsive materials, it is desirable to assemble Janus particles in user-defined environments via user-defined stimuli. However, no general bonding strategy that satisfies these requirements has been demonstrated.
Host–guest interaction could be a general bonding strategy for constructing responsive superstructures of Janus particles. Host–guest interaction is the specific interaction between two or more molecules through supramolecular interactions.5 The flourishing host–guest chemistry provides various types of responsive bonding.5 One well-known responsive host–guest interaction is the interaction between β-cyclodextrin (β-CD) and azobenzene.6 β-CD and trans azobenzene can form host–guest complexes spontaneously. Adding competitive host molecules to the complex can extract azobenzene from β-CD and lead to disassembly of the complex.6a,b Alternatively, UV illumination can also disassemble the complex of azobenzene and β-CD due to photoisomerization of azobenzene.6c,d Responsive host–guest interactions have been utilized to prepare various stimuli-responsive systems, such as vesicles,7 raspberry-like colloids,8 biointerfaces,6c hydrogels,9 and macroscopic assemblies.6a,b,10 However, no attention has been focused on constructing stimuli-responsive superstructures of Janus particles via host–guest interactions.
Herein, we demonstrate the use of responsive host–guest interaction as a new bonding strategy for reversible Janus particle assembly. Janus particles were prepared by depositing gold on one side of sulfate-stabilized polystyrene particles via glancing angle deposition (ESI†). By changing the angle of incidence of the evaporated Au, we obtained three types of Janus particles with different areas of gold patches (Fig. 1). The gold patches for Particle 1, 2, and 3 covered 50 ± 5%, 25 ± 2%, and 7 ± 1% of the total surface area of the particles, respectively. Then, the gold patches were grafted with hydrophilic thiolated β-CD. The Janus particles were discrete in Milli-Q water due to the electrostatic repulsion between particles (Fig. 2b–d left). No aggregation was observed within our experimental time.
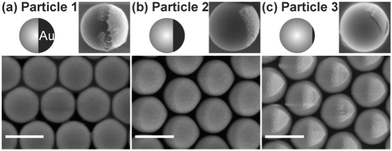 | ||
| Fig. 1 Schematic models and SEM images of three types of Janus particles. The images on the upper right of every panel show typical single particles. The images at the bottom show arrays of Janus particles. The scale bar is 1 μm. Large-area SEM images and more details about fabrication of the Janus particles are provided in ESI.† | ||
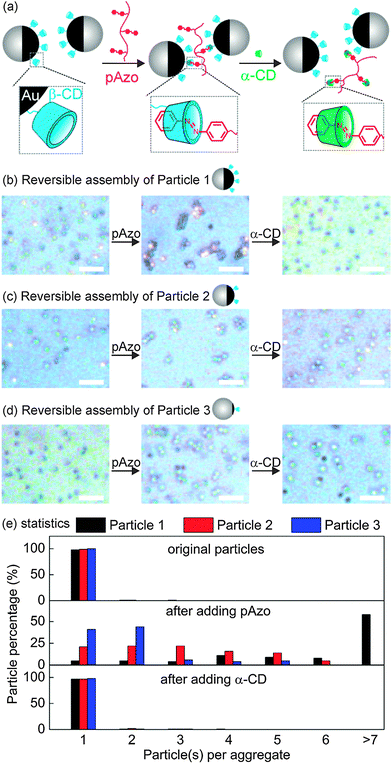 | ||
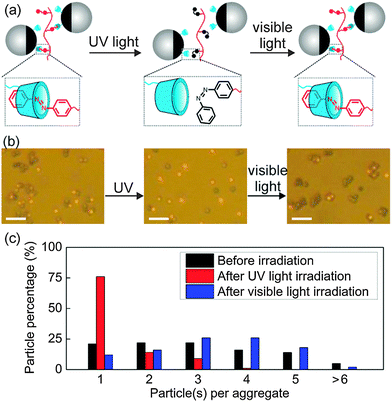
| Fig. 2 (a) Schematic illustration of reversible assembly of β-CD-modified Janus particles by sequential addition of azobenzene-containing polymer (pAzo) and α-CD. (b)–(d) Optical microscopy images: reversible assembly of Particle 1 (b), Particle 2 (c), and Particle 3 (d). The time for assembly and disassembly is 84 s and 105 s, respectively. The scale bar is 5 μm. Corresponding movies are provided in ESI.† (e) Number distribution of Janus particle aggregates consisting of different numbers of particles in the reversible assembly processes. The statistical results are obtained from ∼400 particles. | ||
We synthesized hydrophilic azobenzene-containing polymer pAzo (Mn = ∼5 × 105 g mol−1, Rh = ∼30 nm) by grafting azobenzene groups on poly(acrylic acid) with 3% grafting density (Fig. S1–S4, ESI†). To induce Janus particle assembly, an aqueous solution of pAzo (0.5 mg mL−1, 10 μL) was added to the dispersions of discrete Janus particles (∼109 particles per mL, 10 μL) (Fig. 2b–e middle and Fig. 3). In the dispersion of Particle 1, ∼58% particles assembled into zigzag chains and branched wormlike structures that consisted of more than 7 particles per aggregate. In the dispersion of Particle 2, ∼79% of the particles assembled into small clusters with 2–6 particles per aggregate. In the dispersion of Particle 3, ∼45% of the particles formed dimers. For Particle 1 and 2, the β-CD-modified patch was large enough to accommodate more than one neighbor (Fig. 3 and Fig. S9, ESI†). For Particle 3, the small β-CD-modified patch preferred to accommodate only one neighbor (Fig. 3 and Fig. S9, ESI†). Thus, the number of large aggregates decreased as the area of the β-CD-modified patch decreased. Therefore, different superstructures could be obtained by the assembly of particles with different patches.11
 | ||
| Fig. 3 Confocal laser scanning microscopy images of aggregates of Janus particles: (a) aggregates of Particle 1, (b) aggregates of Particle 2, (c) aggregates of Particle 3. The scale bar is 2 μm. | ||
The Janus particle assembly was also studied in situ using optical microscopy (Movies S1–S3, ESI†). The time scale for Janus particle assembly is seconds to minutes. When pAzo was added, Janus particles began to form dimers and other small clusters. For Particle 1, zigzag chains (2D-like structures) and branched wormlike structures (3D-like structures) were polymerized from small clusters at the later stage of the assembly process. As the size of the aggregates increased, their diffusion slowed down. This may inhibit the further growth of the aggregates of Particle 1 because the further growth of aggregates requires effective collision. For Particle 2 and 3, the size of aggregates was relative small and the aggregates could move freely in a three-dimensional manner. These aggregates could frequently collision. But the small patch size on Particle 2 and 3 may not allow the aggregates to accommodate more neighbors. In addition, the rotation of the Janus particles was observed as the gold patches provide optical contrast by reflecting the light of the optical microscope. Particles still rotated even when particles were part of an aggregate. This rotation indicates that the dynamic feature of the host–guest interaction and the long pAzo chain (Rh = ∼30 nm) allow rotation to occur.
Electrostatic interactions between Janus particles and nonspecific polymer–particle interactions may play a role in self-assembly; adding the polyelectrolyte pAzo to Janus particle dispersion reduced the electrostatic screening length. As a control experiment, we added poly(acrylic acid) without azobenzene groups into the Janus particle dispersion. Approximately 90% of the particles remained discrete (Fig. S5, ESI†). This test verifies that the Janus particles do not assemble by nonspecific polymer–particle interactions or reduction of the electrostatic repulsion. Indeed, Janus particle assembly requires an attractive force between particles. The double-hydrophilic Janus particles with β-CD and sulfate polystyrene on opposite sides could not provide sufficient attraction forces between particles. This control experiment further confirms that the host–guest interaction between β-CD and azobenzene provides an attraction force for Janus particle assembly.
The assembly of Janus particles is reversible. Disassembly was induced by adding an aqueous solution of α-CD (10−2 mol L−1, 10 μL) to the aggregates of Janus particles (Fig. 2b–d right). The association constant between α-CD and azobenzene (2000 M−1) is higher than that between β-CD and azobenzene (770 M−1).9 Disassembly occurred because the competitive host (α-CD) extracts azobenzene from β-CD. The time scale for Janus particle disassembly is seconds to minutes (Movies S4–S6, ESI†). More than 95% of the Janus particles became discrete particles after disassembly for 105 s (Fig. 2e bottom). In a control experiment (Fig. S6, ESI†), the superstructures of the Janus particles were retained when the same amount of Milli-Q water without α-CD was added to the aggregates of Janus particles, which confirms that the disassembly was triggered by α-CD.
The reversible Janus particle assembly could also be controlled by light. Azobenzene exhibits reversible cis–trans photoisomerization (Fig. S7, ESI†). Cis azobenzene has a much weaker association constant with β-CD than does trans azobenzene.9 We prepared aggregates of Janus Particle 2 by adding pAzo to the Janus particle dispersion. Illuminating the aggregates of Particle 2 by UV light (365 nm, 25 mW cm−2, 15 min) induced disassembly of the aggregates (Fig. 4). Approximately 75% of the particles became isolated after UV irradiation. Subsequent visible light irradiation (530 nm, 30 mW cm−2, 15 min) induced Janus particle reassembly.
In conclusion, we demonstrated that Janus particle assembly is reversibly controlled by the host–guest interaction of β-CD and azobenzene. Adding the guest-containing polymer pAzo into the dispersion of host-modified Janus particles induced the assembly of the Janus particles. Zigzag chains, branched wormlike structures, small clusters and dimers of Janus particles were obtained by tuning the patch size of the Janus particles. Disassembly of the superstructures of Janus particles was induced by either adding α-CD or irradiating with UV light. The responsive host–guest interaction is a new bonding strategy for reversible Janus particles assembly. The flourishing host–guest chemistry provides various types of switchable bonds,5 which enable reversible assembly of Janus particles by different stimuli in different environments. The unique features of reversible Janus particle assembly via responsive host–guest interactions provide a novel platform for fabricating stimuli-responsive materials, reconfigurable materials and adaptive optics with various dynamic superstructures.
Y.Z. was supported by the MPG-CAS Joint Doctoral Promotion Programme (DPP). D.W. was supported by the CSC program. This work was partly supported by National Natural Science Foundation of China (No. 21272263).
Notes and references
- P. G. de Gennes, Rev. Mod. Phys., 1992, 64, 645–648 CrossRef.
- (a) S. Jiang, Q. Chen, M. Tripathy, E. Luijten, K. S. Schweizer and S. Granick, Adv. Mater., 2010, 22, 1060–1071 CrossRef CAS PubMed; (b) Q. Chen, J. K. Whitmer, S. Jiang, S. C. Bae, E. Luijten and S. Granick, Science, 2011, 331, 199–202 CrossRef CAS PubMed.
- X. Y. Ling, I. Y. Phang, C. Acikgoz, M. D. Yilmaz, M. A. Hempenius, G. J. Vancso and J. Huskens, Angew. Chem. Int. Edit., 2009, 48, 7677–7682 CrossRef CAS PubMed.
- (a) C. Yu, J. Zhang and S. Granick, Angew. Chem. Int. Edit., 2014, 53, 4364–4367 CrossRef CAS PubMed; (b) L. Feng, R. Dreyfus, R. Sha, N. C. Seeman and P. M. Chaikin, Adv. Mater., 2013, 25, 2779–2783 CrossRef CAS PubMed; (c) Y. Wang, A. D. Hollingsworth, S. K. Yang, S. Patel, D. J. Pine and M. Weck, J. Am. Chem. Soc., 2013, 135, 14064–14067 CrossRef CAS PubMed; (d) S. Sacanna, L. Rossi and D. J. Pine, J. Am. Chem. Soc., 2012, 134, 6112–6115 CrossRef CAS PubMed; (e) J. Yan, K. Chaudhary, S. C. Bae, J. A. Lewis and S. Granick, Nat. Commun., 2013, 4, 1516 CrossRef PubMed; (f) A. Ruditskiy, B. Ren and I. Kretzschmar, Soft Matter, 2013, 9, 9174–9181 RSC.
- See, for example, recent special issue of Responsive Host–Guest Systems in Acc. Chem. Res., 2014, 47, 1923–2234.
- (a) M. Cheng, F. Shi, J. Li, Z. Lin, C. Jiang, M. Xiao, L. Zhang, W. Yang and T. Nishi, Adv. Mater., 2014, 26, 3009–3013 CrossRef CAS PubMed; (b) M. Cheng, Q. Liu, Y. Xian and F. Shi, ACS Appl. Mater. Interfaces, 2014, 6, 7572–7578 CrossRef CAS PubMed; (c) P. B. Wan, Y. P. Wang, Y. G. Jiang, H. P. Xu and X. Zhang, Adv. Mater., 2009, 21, 4362–4365 CrossRef CAS; (d) J. H. Schenkel, A. Samanta and B. J. Ravoo, Adv. Mater., 2014, 26, 1076–1080 CrossRef CAS PubMed.
- Y. Liu, C. Yu, H. Jin, B. Jiang, X. Zhu, Y. Zhou, Z. Lu and D. Yan, J. Am. Chem. Soc., 2013, 135, 4765–4770 CrossRef CAS PubMed.
- Y. Lan, Y. Wu, A. Karas and O. A. Scherman, Angew. Chem., Int. Ed., 2014, 53, 2166–2169 CrossRef CAS PubMed.
- H. Yamaguchi, Y. Kobayashi, R. Kobayashi, Y. Takashima, A. Hashidzume and A. Harada, Nat. Commun., 2012, 3, 603 CrossRef PubMed.
- A. Harada, R. Kobayashi, Y. Takashima, A. Hashidzume and H. Yamaguchi, Nat. Chem., 2011, 3, 34–37 CrossRef CAS PubMed.
- (a) Q. Chen, E. Diesel, J. K. Whitmer, S. C. Bae, E. Luijten and S. Granick, J. Am. Chem. Soc., 2011, 133, 7725–7727 CrossRef CAS PubMed; (b) Q. Chen, S. C. Bae and S. Granick, J. Am. Chem. Soc., 2012, 134, 11080–11083 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4cc09672j |
| This journal is © The Royal Society of Chemistry 2015 |