Open Access Article
Open Access ArticleAntimony-dependent expansion for the Keggin heteropolyniobate family†
Zhe-Yu
Zhang
ab,
Jun
Peng
*a,
Zhen-Yu
Shi
a,
Wan-Li
Zhou
a,
Shifa Ullah
Khan
a and
Hong-Sheng
Liu
*c
aKey Laboratory of Polyoxometalate Science of Ministry of Education, Department of Chemistry, Northeast Normal University, Changchun, Jilin 130024, P. R. China. E-mail: Jpeng@nenu.edu.cn
bDepartment of Chemistry, Baicheng Normal College, Baicheng, Jilin 137000, P. R. China
cSchool of Chemistry and Chemical Engineering, Daqing Normal University, Daqing, Heilongjiang 163712, P. R. China. E-mail: hsliu899@126.com
First published on 2nd January 2015
Abstract
Nine new Sb-bicapped α-Keggin-type heteropolyoxoniobates (HPNb) were synthesized under hydrothermal conditions. Among them, the As-centered HPNb was never reported before, and the two dimer compounds are the biggest isolated HPNbs at present.
Polyoxometalates (POMs), as a large and multifunctional class of discrete nano-sized transition metal oxide clusters, exhibit variety of chemical properties.1 This area is attracting increasing interest for their significant applications in catalysis,2 magnetism3 and nanotechnology.4 As is known, the POM chemistry has been dominated by polyoxo-tungstates, -molybdates and -vanadates. On the other hand, substituted POMs are also a significant branch. In the Nb-substituted POMs, typical examples are the Keggin-type polyoxotungstates, α/β-XW9Nb3O40n− (X = Si, P, Ge or As).5
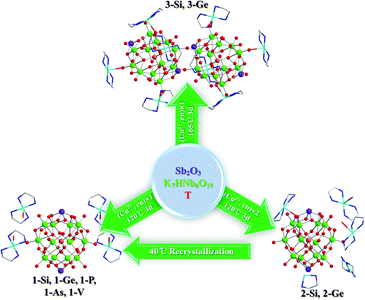
However, a rapid development of Nb-based POM chemistry only occurred in recent decades, because polyoxoniobates (PONbs) were difficult to obtain through the usual acidifications at ambient conditions, as in the case of the classical POMs mentioned above. Pioneer investigations by Nyman, Casey and others, have successfully harvested various isopolyoxoniobates (IPNbs) under basic conditions, including {Nb6},6 {Nb7},7 {Nb10},8 {Nb20},9 {Nb24},10 {Nb27},11 {Nb31}11 and {Nb32}.12 Relatively, heteropolyniobate (HPNb) chemistry is explored rarely, perhaps due to severe reaction conditions, such as higher temperatures and narrow pH windows (10.5–12.5). Since Nyman and coworkers reported the first HPNbs, [Ti2O2][SiNb12O40]12− and [H2Si4Nb16O56]14−,13 a dozen of the Keggin-type HPNbs and analogues have been reported, namely, [XNb18O54]n− (X = Si, Ga or Al),14 [XNb12O40]16− (X = Si or Ge),15 [XNb12O40(VO)2]n− (X = Si, Ge, P or V),16 [(PO2)3PNb9O34]15−,17 [Nb2O2][TNb12O40]10−,18 [Nb2O2(H2O)2][SiNb12O40]10−,19 [X2(XOH)2Nb16O54]14− (X = Si or Ge),20 [XNb8V4O40(VO)4]15− (X = P or V) and [PNb12O40(VO)6]3−.21 Among these HPNbs, most possess the capped Keggin-type structures, with {TiOn},13 {VO}16,21 and {NbOn}18,19 as the capping groups, to neutralize high negative charges of Keggin cores. Inspired by the foregoing work, we tried to introduce Sb into the HPNb system, as Sb3+ ion with high positive charge, may play a capping role anchoring on the HPNb clusters to neutralize the Keggin cores. Till now, only Sb-bicapped Keggin-type {PMo12O40Sb2} cores were reported by Xu's group,22 and [PNb12Sb2O40]n− was reported by Casey's group quite recently.23 Herein, we report a series of Sb-bicapped HPNb compounds, [Cu(en)2(H2O)2]4[α-HnTNb12O40Sb2]·18H2O (1) [T: Si, n = 2 (1-Si); Ge, n = 2 (1-Ge); P, n = 1 (1-P); As, n = 1 (1-As), V, n = 1 (1-V)]; [Cu(en)2]3 [Cu(en)2(H2O)]4{[Cu(en)2]2[α-HTNb12O40Sb2]2}·18H2O (2) [T: Si (2-Si); Ge (2-Ge)]; [Cu(en)2]{[Cu(en)2]3[α-TNb12O39Sb2]}·11H2O (3) [T: Si (3-Si); Ge (3-Ge)] (en = ethanediamine). All these compounds consist of new Sb-bicapped [TNb12O40Sb2]n− polyanions. Especially, the As-centered Keggin-type HPNb cluster was never reported before. 3 is the first example of dimer fused by two Keggin-type HPNb clusters, which are the biggest isolated clusters based on the Keggin-HPNb at present.
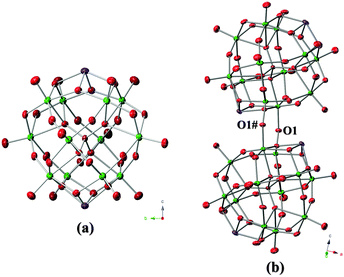
The single-crystal X-ray diffraction analysis revealed that five compounds of 1 were isostructural, thus the structure of 1-Si is described in details. The cell unit of 1-Si consisted a quarter of a crystallographically independent Sb-bicapped Keggin-type polyanion [α-H2SiNb12O40Sb2]8− (as shown in Fig. 1a) and one crystallographically independent [Cu(en)2(H2O)]2+ cation. Two Sb atoms, in a distorted square pyramidal coordination geometry oppositely capped on two {Nb4O4} (O6, O7, O6#, O7#) windows of the {α-SiNb12} core (Fig. S1, ESI†). Sb–O bond lengths and O–Sb–O bond angles were in the ranges of 1.992(6)–2.152(6) Å and 76.3(2)–135.8(3)°, respectively. Thus, the symmetry of the α-Keggin-type cluster was lowered from Td to D2d, and the negative charge of the Keggin HPNb anion was neutralized from −14 in an uncapped one to −8 in the capped one. [Cu(en)2(H2O)]2+ was a free cation in which Cu2+ was chelated by two en molecules and coordinated by one water molecule in a tetragonal pyramid coordination geometry. The bond parameters for 1 are listed in Tables S3, S4 and S6 (ESI†).
The two compounds of 2 were isostructural, comprised of one crystallographically independent [HTNb12O40Sb2]9−, two crystallographically independent [Cu(en)2(H2O)]2+ (Cu1 and Cu4) and 2.5 crystallographically independent [Cu(en)2]2+ (Cu2, Cu3 and Cu5) (Fig. S2, ESI†). The bond parameters for 2 are listed in Tables S5, S8 and S9 (ESI†).
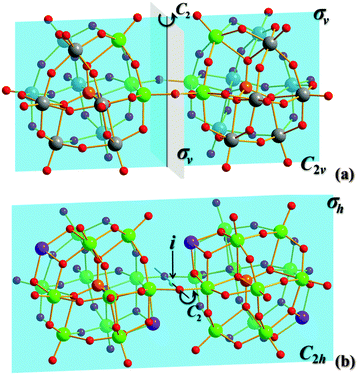
The two compounds of 3 were isostructural. Each one consists of an unusual dimer [(TNb12O38Sb2)2(μ2-O)2]16− anion formed by two Keggin-type {α-TNb12Sb2} clusters via two μ2-O (O1 and O1#) (Fig. 1b). The Nb–μ2-O bond lengths and Nb–μ2-O–Nb bond angles for 3 are as shown in Fig. S3 (ESI†). The dimer has a C2hanti symmetry with a symmetric center coinciding with the center of O1⋯O1#, a symmetric axis passing through O1⋯O1#, and a mirror plane of Sb1, Sb2, Sb1# and Sb2#. The C2hanti symmetry, first found in the structures of the Keggin-type HPNbs, was different from the C2vsyn symmetry of the [(SiW9Nb3O38)2(μ2-O)2]10− core reported by Hill (Fig. 2).5b In the unit cell, three [Cu(en)2]2+ fragments decorated a {TNb12O39Sb2} cluster via weak coordination interactions between Cu and Ot (Ot, terminal oxygen) of a HPNb core to form a tri-supporting HPNb cluster (Fig. S4, ESI†). The Cu–O bond lengths for 3-Si were 2.346(6) Å (Cu1–O3), 2.269(6) Å (Cu2–O12) and 2.378(6) Å (Cu3–O7), and the corresponding Cu–O bond lengths for 3-Ge were 2.381(6) Å, 2.302(6) Å and 2.500 Å, respectively. The bond parameters of 3 are listed in Tables S7, S10 and S11 (ESI†).
All the compounds were obtained under hydrothermal conditions. Scheme 1 shows the synthetic process, and the experimental details are described in ESI.† Reaction time, temperature and proportion of starting materials are important factors. When double amount of Cu(CH3COO)2 and en were added, compounds of 2 were obtained with a little amount of 1. In addition, when 2 were recrystallized, corresponding compounds of 1 were obtained. Interestingly, crystals of 1 were able to be isolated not merely through the usual “one-pot hydrothermal synthesis procedure”, but also through a two-step route, that was, the starting materials without Cu and en firstly suffered a hydrothermal reaction, later Cu2+ and en were added into the hydrothermal-reacted solution. It means that the generation of {TNb12O40Sb2} clusters was independent of Cu2+ and en, and [Cu(en)2(H2O)]2+ just plays a counterion role. Compounds of 3 were isolated at 165 °C, a temperature that is 45 °C higher than that for 1, and we therefore deduced that a higher temperature was favorable for the dimerization of {TNb12O40Sb2} clusters. What's more important is that Sb(III) plays a key role to stabilize the Keggin core by neutralizing its excess negative charge.
In summary, nine new HPNb compounds were synthesized in the presence of Sb(III) by different routes, and the HPNb family obtained an expansion. All the compounds were based on the Sb-bicapped Keggin clusters. Especially, 1-As was the first As-centered Keggin-type HPNb compound. Temperature is a key factor for the dimerization of {TNb12O40Sb2} to form the {(TNb12O38Sb2)2(μ2-O)2} dimer. The dimer presents three significant features: (1) its C2hanti symmetry is firstly found in the structures of the Keggin-type HPNbs; (2) it is the first example of a dimer fused by the Keggin-type HPNbs; (3) it possesses the highest nuclearity in the isolated HPNb clusters at present. In the following work, we will explore more capping-groups to extend the HPNb family.
We thank the National Natural Science Foundation of China (grant 21373044 and 21171030) and National Natural Science Foundation of Heilongjiang Province (B201012) for financial supports.
Notes and references
- (a) A. Dolbecq, E. Dumas, C. R. Mayer and P. Mialane, Chem. Rev., 2010, 110, 6009–6048 CrossRef CAS PubMed; (b) P. Yin, D. Li and T. Liu, Chem. Soc. Rev., 2012, 41, 7368–7383 RSC.
- K. Suzuki, F. Tang, Y. Kikukawa, K. Yamaguchi and N. Mizuno, Angew. Chem., Int. Ed., 2014, 53, 5356–5360 CrossRef CAS PubMed.
- (a) J. D. Compain, P. Mialane, A. Dolbecq, I. M. Mbomekalle, J. Marrot, F. Secheresse, E. Riviere, G. Rogez and W. Wernsdorfer, Angew. Chem., Int. Ed., 2009, 48, 3077–3081 CrossRef CAS PubMed; (b) J. M. Clemente-Juan, E. Coronado and A. Gaita-Arino, Chem. Soc. Rev., 2012, 41, 7464–7478 RSC; (c) R. Al-Oweini, B. S. Bassil, J. Friedl, V. Kottisch, M. Ibrahim, M. Asano, B. Keita, G. Novitchi, Y. Lan, A. Powell, U. Stimming and U. Kortz, Inorg. Chem., 2014, 53, 5663–5673 CrossRef CAS PubMed.
- D. Ma, L. Liang, W. Chen, H. Liu and Y.-F. Song, Adv. Funct. Mater., 2013, 23, 6100–6105 CrossRef CAS.
- (a) R. G. Finke and M. W. Droege, J. Am. Chem. Soc., 1984, 106, 7274–7277 CrossRef CAS; (b) G.-S. Kim, H. Zeng, W. A. Neiwert, J. J. Cowan, D. VanDerveer, C. L. Hill and I. A. Weinstock, Inorg. Chem., 2003, 42, 5537–5544 CrossRef CAS PubMed; (c) S. J. Li, S. X. Liu, C. C. Li, F. J. Ma, D. D. Liang, W. Zhang, R. K. Tan, Y. Y. Zhang and L. Xu, Chem. – Eur. J., 2010, 16, 13435–13442 CrossRef CAS PubMed; (d) S.-J. Li, S.-X. Liu, C.-C. Li, F.-J. Ma, W. Zhang, D.-D. Liang, R.-K. Tan, Y.-Y. Zhang and Q. Tang, Inorg. Chim. Acta, 2011, 376, 296–301 CrossRef CAS PubMed.
- (a) A. V. Besserguenev, M. H. Dickman and M. T. Pope, Inorg. Chem., 2001, 40, 2582–2586 CrossRef CAS PubMed; (b) R. P. Bontchev, E. L. Venturini and M. Nyman, Inorg. Chem., 2007, 46, 4483–4491 CrossRef CAS PubMed.
- J. Niu, P. Ma, H. Niu, J. Li, J. Zhao, Y. Song and J. Wang, Chem. – Eur. J., 2007, 13, 8739–8748 CrossRef CAS PubMed.
- (a) E. M. Villa, C. A. Ohlin, E. Balogh, T. M. Anderson, M. D. Nyman and W. H. Casey, Angew. Chem., Int. Ed., 2008, 47, 4844–4846 CrossRef CAS PubMed; (b) C. A. Ohlin, E. M. Villa, J. C. Fettinger and W. H. Casey, Dalton Trans., 2009, 2677–2678 RSC.
- M. Maekawa, Y. Ozawa and A. Yagasaki, Inorg. Chem., 2006, 45, 9608–9609 CrossRef CAS PubMed.
- R. P. Bontchev and M. Nyman, Angew. Chem., Int. Ed., 2006, 45, 6670–6672 CrossRef CAS PubMed.
- R. Tsunashima, D. L. Long, H. N. Miras, D. Gabb, C. P. Pradeep and L. Cronin, Angew. Chem., Int. Ed., 2010, 49, 113–116 CrossRef CAS PubMed.
- P. Huang, C. Qin, Z. M. Su, Y. Xing, X. L. Wang, K. Z. Shao, Y. Q. Lan and E. B. Wang, J. Am. Chem. Soc., 2012, 134, 14004–14010 CrossRef CAS PubMed.
- M. Nyman, F. Bonhomme, T. M. Alam, M. A. Rodriguez, B. R. Cherry, J. L. Krumhansl, T. M. Nenoff and A. M. Sattler, Science, 2002, 297, 996–998 CrossRef CAS PubMed.
- (a) Y. Hou, M. Nyman and M. A. Rodriguez, Angew. Chem., Int. Ed., 2011, 50, 12514–12517 CrossRef CAS PubMed; (b) Y. Hou, T. M. Alam, M. A. Rodriguez and M. Nyman, Chem. Commun., 2012, 48, 6004–6006 RSC.
- M. Nyman, F. Bonhomme, T. M. Alam, J. B. Parise and G. M. Vaughan, Angew. Chem., Int. Ed., 2004, 43, 2787–2792 CrossRef CAS PubMed.
- (a) G. Guo, Y. Xu, J. Cao and C. Hu, Chem. Commun., 2011, 47, 9411–9413 RSC; (b) J. H. Son, C. A. Ohlin, R. L. Johnson, P. Yu and W. H. Casey, Chem. – Eur. J., 2013, 19, 5191–5197 CrossRef CAS PubMed; (c) Y. Zhang, J.-Q. Shen, L.-H. Zheng, Z.-M. Zhang, Y.-X. Li and E.-B. Wang, Cryst. Growth Des., 2013, 14, 110–116 CrossRef.
- M. Nyman, A. J. Celestian, J. B. Parise, G. P. Holland and T. M. Alam, Inorg. Chem., 2006, 45, 1043–1052 CrossRef CAS PubMed.
- F. Bonhomme, J. P. Larentzos, T. M. Alam, E. J. Maginn and M. Nyman, Inorg. Chem., 2005, 44, 1774–1785 CrossRef CAS PubMed.
- Z. Zhang, Q. Lin, D. Kurunthu, T. Wu, F. Zuo, S. T. Zheng, C. J. Bardeen, X. Bu and P. Feng, J. Am. Chem. Soc., 2011, 133, 6934–6937 CrossRef CAS PubMed.
- (a) T. M. Anderson, T. M. Alam, M. A. Rodriguez, J. N. Bixler, W. Xu, J. B. Parise and M. Nyman, Inorg. Chem., 2008, 47, 7834–7839 CrossRef CAS PubMed; (b) X. Zhang, S.-X. Liu, S.-J. Li, Y.-H. Gao, X.-N. Wang, Q. Tang and Y.-W. Liu, Eur. J. Inorg. Chem., 2013, 1706–1712 CrossRef CAS.
- (a) J. Q. Shen, Q. Wu, Y. Zhang, Z. M. Zhang, Y. G. Li, Y. Lu and E. B. Wang, Chem. – Eur. J., 2014, 20, 2840–2848 CrossRef CAS PubMed; (b) J. Q. Shen, Y. Zhang, Z. M. Zhang, Y. G. Li, Y. Q. Gao and E. B. Wang, Chem. Commun., 2014, 50, 6017–6019 RSC.
- (a) Q.-B. Zhang, Y.-K. Lu, Y.-B. Liu, J. Lu, M.-H. Bi, J.-H. Yu, T.-G. Wang, J.-Q. Xu and J. Liu, Inorg. Chem. Commun., 2006, 9, 544–547 CrossRef CAS PubMed; (b) S. Y. Shi, Y. Wang, X. B. Cui, G. W. Wang, G. D. Yang and J. Q. Xu, Dalton Trans., 2009, 6099–6102 RSC.
- J. H. Son and W. H. Casey, Chem. Commun., 2015, 51, 1436–1438 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis, details of crystallographic data, structural figures, tables of selected bond lengths and bond angles, BVS calculation results of V and Sb atoms, TGA, XRPD, IR, UV-vis and EDX. CCDC 971581, 1020912–1020914, 1025243, 1034780–1034783. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4cc09612f |
| This journal is © The Royal Society of Chemistry 2015 |