Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
On-chip monitoring of chemical syntheses in microdroplets via surface-enhanced Raman spectroscopy†
T.-A.
Meier
,
R. J.
Beulig
,
E.
Klinge
,
M.
Fuss
,
S.
Ohla
and
D.
Belder
*
Institut für Analytische Chemie, Universität Leipzig, Linnéstr. 3, D-04103 Leipzig, Germany. E-mail: belder@uni-leipzig.de
First published on 12th January 2015
Abstract
Conducting organic syntheses in microfluidic chips allows studying and optimising chemical reactions at minimal time-scales and resource consumption. Herein, we describe a multi-channel microdroplet chip, which allows fast and directed dispensing of reactants into individual droplets in a segmented flow. This gives access to study the reaction progress in situ via surface-enhanced Raman spectroscopic monitoring of fast moving individual droplets. This opens up new avenues for high-throughput screening of organic reactions at the micro- and nano-scale.
Microfluidic systems are attractive tools in various areas of chemistry1 and life sciences.2 They enable us to study chemical processes with minimal resource consumption at high speed and throughput, which is useful for screening and fast optimisation of chemical syntheses.3 Chip-based systems are valuable as they can provide seamless integration of different functionalities within a small space,4e.g. the combination of microflow reactions with continuous product separation.5
In microfluidic chips with a segmented flow single droplets can serve as individual reaction containers. Due to the low sample consumption and high process speed such microfluidic devices are very attractive for high throughput experiments6 with precious compounds.
The analytical monitoring of chemical processes in fast moving nanoliter-droplets is, however, challenging.7 Fluorescence detection, which is quite common in bio-analytics,8 is easy to realise, yet only applicable for processes containing luminescent substances. An interesting alternative with a much wider scope is the coupling of microfluidic droplet chips to mass spectrometry.9 A strong point of such chip-MS coupling is the more reliable compound identification with the aid of the acquired mass spectra.10 A disadvantage is, however, that mass spectrometric detection is hardly compatible with further downstream processes due to the destructive analytical procedure.
In this context, Raman spectroscopy provides an interesting approach for non-destructive analytical characterization in microfluidic chips which can provide structural information.11 In comparison with IR-spectroscopy it is less demanding with regard to the compatibility with chip materials and process media.12
The rather low sensitivity of Raman scattering can be faced using surface enhanced Raman spectroscopy (SERS). The interaction with rough noble metal surfaces can dramatically enhance the analyte’s Raman signals.13 Silver nanoparticles are known to be excellent substrates for this purpose.14 They can be added straightforwardly to the solution of interest, which is very appealing for the application in chip-based droplet microfluidics.15
While almost all publications on SERS-detection to date have covered either biological/biochemical processes or studies on model substances like crystal violet or rhodamine 6G,16 this article focuses on time-resolved investigation of an organic-chemical synthesis in a segmented micro-scaled flow. A challenge is the short acquisition time, if the dynamic processes shall be monitored on-the-fly in single droplets as well as the controlled dosing of reagents including the SERS-colloidal solution at the micro- and nano-scale.
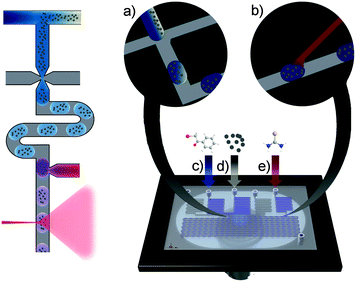
We introduce a microfluidic system, which allows studying the scope of organic syntheses at the nanoliter scale by Raman spectroscopic monitoring of individual fast moving droplets. For this purpose we developed a chip layout, presented in Fig. 1 which contains a flow-focussing structure (Fig. 1a), where 5 nL droplets are generated with a frequency of 2 Hz. These are transported downstream in a hydrophobic carrier fluid (Fluorinert FC-40) towards a dispensing structure (Fig. 1b). At that point another aqueous liquid, containing a reactant and/or a nanoparticle suspension, is added. This is followed by a serpentine track where the reaction can take place. The fluid transport is driven by syringe pumps.
Prototypes of the chips were fabricated from polydimethylsiloxane (PDMS)/glass using common moulding techniques.17 The resulting channels had a height of 106.4 ± 1.1 μm and a width of 92.3 ± 3.6 μm. The serpentine track was about 44.5 cm long and the outer dimensions of the chip were about 43 × 10 mm. In order to obtain channel surfaces with uniform hydrophobicity the channels were treated with the commercial available hydrophobicity agent Rain-X® and flushed with isopropanol afterwards.18 This suppressed wetting of the channel walls by the aqueous droplets, and significantly improved the droplet-stability and robustness of the whole process.
This system allows following chemical reactions inside individual microdroplets by a directed addition of silver colloids to each droplet.
To evaluate and demonstrate the performance of the approach we chose the Hantzsch-synthesis of 2-aminothiazoles as a model system.19 Initially, we studied and optimised the reaction off-chip, where we found that the reaction towards 2-amino-4-phenylthiazole is sufficiently fast using an ethanol–water-solution (8.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5; v/v) as solvent. This process was followed by ESI-mass spectrometry as well as by surface enhanced Raman spectroscopy on a microscope slide after adding a silver nanoparticle suspension to the reaction solution. The Raman- and the MS-data on monitoring the reaction progress were in good agreement supporting the feasibility of the concept (Fig. S1 and S2, ESI†).
1.5; v/v) as solvent. This process was followed by ESI-mass spectrometry as well as by surface enhanced Raman spectroscopy on a microscope slide after adding a silver nanoparticle suspension to the reaction solution. The Raman- and the MS-data on monitoring the reaction progress were in good agreement supporting the feasibility of the concept (Fig. S1 and S2, ESI†).
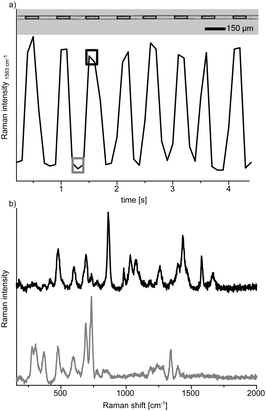
Thereafter the process was transferred to the chip with the intention to study the reaction progress in individual droplets within a segmented flow. For that purpose the bromoacetophenone solution and the silver nanoparticle suspension were brought together via discrete microchannels in front of the droplet generator and thiourea was added downstream. After careful optimisation of the flow rates to 0.2 μL min−1 for the aqueous solutions and 0.25 μL min−1 for the carrier fluids we obtained a stable segmented flow although the high ethanol content slightly hampered the process. Occasionally, we observed, however, minor variances in the spacing between droplets as evident from Fig. 2a. This experimental approach gives access to SERS-spectra right after reaction initiation and is ideally suited for kinetic studies. The reaction progress can be monitored by following the moving droplets downstream, along the serpentine acquiring SERS-spectra from individual droplets. In order to achieve adequate signal-to-noise ratios we chose an acquisition time of 500 ms which allowed to monitor individual passing droplet as well as the carrier fluid.
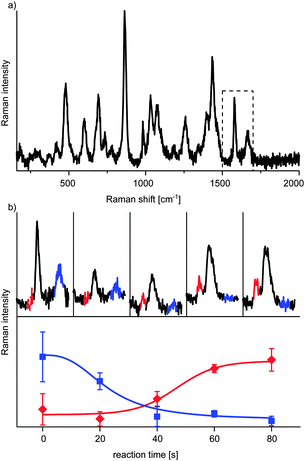
In the present study, we chose five detection positions along the channel which represent different reaction times. The first one was placed at the reaction starting point and the following were located downstream in a way that the flow rate dependent residence time between each position was about 20 s. The microfluidic chip was placed on top of a Raman microscope and spectra were taken at every detection position from the droplets as well as from the carrier fluid (Fig. 2). As spectra from inside and outside the droplets differ significantly, this allows straightforward monitoring of the segmented flow. The baseline was stable over the period of investigation (about two hours) revealing the absence of substrate dispersion or adsorption to the channel wall (Fig. S3, ESI†). Spectroscopic tracing of the reactants was accomplished via indicator bands, determined in the above mentioned off-chip experiments (Fig. 2). 2-Bromoacetophenone for example shows strong bands at 1583 cm−1 and 1665 cm−1 (Fig. 3a), which do not appear in the product spectrum. The spectrum of 2-amino-4-phenylthiazole, however, has strong bands at 1551 cm−1 and 1593 cm−1, which are absent in the reactant spectrum. The reaction process can now be monitored by changes of the relative intensities of these bands. For a more reliable data acquisition, the solvent band at 863 cm−1 was used as internal standard to normalise the spectra.
With this approach we could observe that the band of the starting material at 1665 cm−1 decreased to a minimum within 80 s, while the product band at 1551 cm−1 increased and reached a plateau within the same time (Fig. 3b). These data prove that the system is indeed capable to follow organic reactions with SERS inside fast moving single droplets.
An interesting application of the approach is in the area for rapid optimisation of reaction conditions or for studying the scope of reactions testing various substrates. The latter is demonstrated in this proof of concept study by the use of various substrates for the Hantzsch-synthesis. This can be elegantly realised on chip in a single experiment by using different reagent channels or more simple as in the present study by using various substrate solutions in a sequential manner. We tested nine different substrates and performed the respective syntheses as described above; the results are summarized in Table 1. In order to follow the reactions with the various reagents spectroscopically we had to choose appropriate Raman bands. For example products made from N-methylthiourea did not show the NH-band at 1551 cm−1 and an indicator-band at 1593 cm−1 was used alternatively (Fig. S4, ESI†).
| a ↑ = increasing band intensity, ↓ = decreasing band intensity, × = band is absent → = band intensity stays constant. | |||
|---|---|---|---|
|
Ar = C6H5,
R3 = Ha |
Ar = p-F–C6H5,
R3 = H |
Ar = C6H5,
R3 = CH3 |
|
|
R1 = H,
R2 = H |
1583 ↓; 1665 ↓
1551 ↑; 1593 ↑ |
1583 ↓; 1665 ↓ 1551 ↑; 1593 ↑ | 1583 →; 1665 → 1551 ×; 1593 × |
| R1 = H, R2 = CH3 |
1583 ↓; 1665 ↓
1551 ×; 1593 ↑ |
1583 ↓; 1665 ↓ 1551 ×; 1593 ↑ | 1583 →; 1665 → 1551 ×; 1593 × |
| R1 = CH3, R2 = CH3 |
1583 →; 1665 ↓
1551 ×; 1593 × |
1583 →; 1665 ↓ 1551 ×; 1593 × | 1583 →; 1665 → 1551 ×; 1593 × |
The experimental design presented above allows studying the progress of reactions at high speed with minimal sample consumption. For enabling SERS detection we had to add silver colloids to the reaction solution which could of course interfere with the desired reaction and lead to misinterpretations. This can be avoided if the silver colloid is added downstream at the end of the reaction, immediately before the detection point. In order to test if the addition of the silver colloid solution affected the reaction, we modified the experiment accordingly and infused the silver colloid solution from the inlet (e) shown in Fig. 1. The quite similar results for a reaction performed if the colloid solution is added subsequently to the reaction solution can be seen in Fig. S5 (ESI†).
In summary, we introduce an approach for studying chemical reactions in individual fast moving droplets by surface-enhanced Raman spectroscopy. We developed a microfluidic droplet chip manufactured from PDMS/glass via moulding and plasma initiated bonding. The design allows a precise control of droplet conditions and reliable dosing of reactants and silver colloids. The chemical process was monitored in real time via surface-enhanced Raman spectroscopy using silver nanoparticles as reporter substrates. In a proof of concept study we could successfully apply it to follow the syntheses of 2-aminothiazoles. The developed approach is ideally suited for studying chemical reactions on the nanoliter scale.
Notes and references
- (a) G. M. Whitesides, Nature, 2006, 442, 368 CrossRef CAS PubMed; (b) A. Arora, G. Simone, G. B. Salieb-Beugelaar, J. T. Kim and A. Manz, Anal. Chem., 2010, 82, 4830 CrossRef CAS PubMed; (c) R. R. Pompano, W. Liu, W. Du and R. F. Ismagilov, Annu. Rev. Anal. Chem., 2011, 4, 59 CrossRef CAS PubMed; (d) D. Mark, S. Haeberle, G. Roth, F. von Stetten and R. Zengerle, Chem. Soc. Rev., 2010, 39, 1153 RSC.
- (a) C. T. Culbertson, T. G. Mickleburgh, S. A. Stewart-James, K. A. Sellens and M. Pressnall, Anal. Chem., 2013, 86, 95 CrossRef PubMed; (b) H. N. Joensson and H. Andersson Svahn, Angew. Chem., Int. Ed., 2012, 51, 12176 CrossRef CAS PubMed.
- (a) S. Nagl, P. Schulze, S. Ohla, R. Beyreiss, L. Gitlin and D. Belder, Anal. Chem., 2011, 83, 3232 CrossRef CAS PubMed; (b) S. Ehlert and U. Tallarek, Anal. Bioanal. Chem., 2007, 388, 517–520 CrossRef CAS PubMed; (c) J. P. Kutter, J. Chromatogr. A, 2012, 1221, 72 CrossRef CAS PubMed; (d) P. Jáč and G. K. E. Scriba, J. Sep. Sci., 2013, 36, 52 CrossRef PubMed; (e) S. Ohla, R. Beyreiss, S. Fritzsche, P. Glaser, S. Nagl, K. Stockhausen, C. Schneider and D. Belder, Chem. – Eur. J., 2012, 18, 1240 CrossRef CAS PubMed; (f) D. T. McQuade and P. H. Seeberger, J. Org. Chem., 2013, 78, 6384 CrossRef CAS PubMed; (g) D. Janasek, J. Franzke and A. Manz, Nature, 2006, 442, 374 CrossRef CAS PubMed.
- (a) L. Gitlin, C. Hoera, R. J. Meier, S. Nagl and D. Belder, Lab Chip, 2013, 13, 4134 RSC; (b) D. Belder, Angew. Chem., Int. Ed., 2009, 48, 3736 CrossRef CAS PubMed; (c) O. Trapp, S. K. Weber, S. Bauch and W. Hofstadt, Angew. Chem., Int. Ed., 2007, 46, 7307 CrossRef CAS PubMed; (d) I. R. Baxendale, J. Chem. Technol. Biotechnol., 2013, 88, 519 CrossRef CAS; (e) A. J. deMello, Nature, 2006, 442, 394 CrossRef CAS PubMed; (f) R. L. Hartman, J. P. McMullen and K. F. Jensen, Angew. Chem., Int. Ed., 2011, 50, 7502 CrossRef CAS PubMed; (g) C. Wiles and P. Watts, Chem. Commun., 2011, 47, 6512 RSC; (h) J. Yue, J. C. Schouten and T. A. Nijhuis, Ind. Eng. Chem. Res., 2012, 51, 14583 CrossRef CAS.
- (a) S. Jezierski, V. Tehsmer, S. Nagl and D. Belder, Chem. Commun., 2013, 49, 11644 RSC; (b) C. Benz, M. Boomhoff, J. Appun, C. Schneider and D. Belder, Angew. Chem., Int. Ed., 2015 DOI:10.1002/anie.201409663R2.
- (a) J. H. Kim, T. Y. Jeon, T. M. Choi, T. S. Shim, S.-H. Kim and S.-M. Yang, Langmuir, 2013, 30, 1473 CrossRef PubMed; (b) C. E. Stanley, R. C. R. Wootton and A. J. deMello, Chimia, 2012, 66, 88 CrossRef CAS PubMed; (c) H. Song, J. D. Tice and R. F. Ismagilov, Angew. Chem., Int. Ed., 2003, 42, 768 CrossRef CAS PubMed; (d) A. B. Theberge, F. Courtois, Y. Schaerli, M. Fischlechner, C. Abell, F. Hollfelder and W. T. S. Huck, Angew. Chem., Int. Ed., 2010, 49, 5846 CrossRef CAS PubMed.
- Y. Zhu and Q. Fang, Anal. Chim. Acta, 2013, 787, 24 CrossRef CAS PubMed.
- (a) P. Dittrich and A. Manz, Anal. Bioanal. Chem., 2005, 382, 1771–1782 CrossRef CAS PubMed; (b) C. Benz, H. Retzbach, S. Nagl and D. Belder, Lab Chip, 2013, 13, 2808 RSC; (c) S. Götz and U. Karst, Anal. Bioanal. Chem., 2007, 387, 183–192 CrossRef PubMed; (d) K. B. Mogensen and J. P. Kutter, Electrophoresis, 2009, 30, S92 CrossRef PubMed.
- (a) S. K. Küster, S. R. Fagerer, P. E. Verboket, K. Eyer, K. Jefimovs, R. Zenobi and P. S. Dittrich, Anal. Chem., 2013, 85, 1285 CrossRef PubMed; (b) L. M. Fidalgo, G. Whyte, B. T. Ruotolo, J. L. P. Benesch, F. Stengel, C. Abell, C. V. Robinson and W. T. S. Huck, Angew. Chem., Int. Ed., 2009, 48, 3665 CrossRef CAS PubMed.
- (a) S. Ohla and D. Belder, Curr. Opin. Chem. Biol., 2012, 16, 453 CrossRef CAS PubMed; (b) F. Schwarzkopf, T. Scholl, S. Ohla and D. Belder, Electrophoresis, 2014, 1 Search PubMed.
- (a) A. F. Chrimes, K. Khoshmanesh, P. R. Stoddart, A. Mitchell and K. Kalantar-zadeh, Chem. Soc. Rev., 2013, 42, 5880 RSC; (b) G. Chaplain, S. J. Haswell, P. D. I. Fletcher, S. M. Kelly and A. Mansfield, Aust. J. Chem., 2013, 66, 208 CrossRef CAS; (c) M. Lee, J.-P. Lee, H. Rhee, J. Choo, Y. Gyu Chai and E. Kyu Lee, J. Raman Spectrosc., 2003, 34, 737 CrossRef CAS; (d) L. Chen and J. Choo, Electrophoresis, 2008, 29, 1815 CrossRef CAS PubMed; (e) R. Keir, E. Igata, M. Arundell, W. E. Smith, D. Graham, C. McHugh and J. M. Cooper, Anal. Chem., 2002, 74, 1503 CrossRef CAS.
- (a) S. E. Barnes, Z. T. Cygan, J. K. Yates, K. L. Beers and E. J. Amis, Analyst, 2006, 131, 1027 RSC; (b) M. Dorobantu Bodoc, L. Prat, C. Xuereb, C. Gourdon and T. Lasuye, Chem. Eng. Technol., 2012, 35, 705 CrossRef CAS; (c) P. J. Aarnoutse and J. A. Westerhuis, Anal. Chem., 2005, 77, 1228 CrossRef CAS PubMed; (d) S. Mozharov, A. Nordon, J. M. Girkin and D. Littlejohn, Lab Chip, 2010, 10, 2101 RSC; (e) V. Joseph, C. Engelbrekt, J. Zhang, U. Gernert, J. Ulstrup and J. Kneipp, Angew. Chem., Int. Ed., 2012, 51, 7592 CrossRef CAS PubMed; (f) P. D. I. Fletcher, S. J. Haswell and X. Zhang, Electrophoresis, 2003, 24, 3239 CrossRef CAS PubMed.
- (a) M. Fleischmann, P. J. Hendra and A. J. McQuillan, Chem. Phys. Lett., 1974, 26, 163 CrossRef CAS; (b) L. Opilik, T. Schmid and R. Zenobi, Annu. Rev. Anal. Chem., 2013, 6, 379 CrossRef CAS PubMed; (c) S. Schlücker, Angew. Chem., Int. Ed., 2014, 53, 4756 CrossRef PubMed.
- (a) S. Nie and S. R. Emory, Science, 1997, 275, 1102 CrossRef CAS PubMed; (b) K. Kneipp, Y. Wang, H. Kneipp, L. T. Perelman, I. Itzkan, R. R. Dasari and M. S. Feld, Phys. Rev. Lett., 1997, 78, 1667 CrossRef CAS.
- (a) K. R. Strehle, D. Cialla, P. Rösch, T. Henkel, M. Köhler and J. Popp, Anal. Chem., 2007, 79, 1542 CrossRef CAS PubMed; (b) K. R. Ackermann, T. Henkel and J. Popp, ChemPhysChem, 2007, 8, 2665 CrossRef CAS PubMed; (c) G. Cristobal, L. Arbouet, F. Sarrazin, D. Talaga, J.-L. Bruneel, M. Joanicot and L. Servant, Lab Chip, 2006, 6, 1140 RSC; (d) C. D. Syme, C. Martino, R. Yusvana, N. M. S. Sirimuthu and J. M. Cooper, Anal. Chem., 2011, 84, 1491 CrossRef PubMed; (e) R. Gao, N. Choi, S.-I. Chang, S. H. Kang, J. M. Song, S. I. Cho, D. W. Lim and J. Choo, Anal. Chim. Acta, 2010, 681, 87 CrossRef CAS PubMed.
- A. März, T. Henkel, D. Cialla, M. Schmitt and J. Popp, Lab Chip, 2011, 11, 3584 RSC.
- L. Gitlin, P. Schulze and D. Belder, Lab Chip, 2009, 9, 3000 RSC.
- I. Sinn, P. Kinnunen, T. Albertson, B. H. McNaughton, D. W. Newton, M. A. Burns and R. Kopelman, Lab Chip, 2011, 11, 2604 RSC.
- T. M. Potewar, S. A. Ingale and K. V. Srinivasan, Tetrahedron, 2008, 64, 5019 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and supporting measurements. See DOI: 10.1039/c4cc09595b |
| This journal is © The Royal Society of Chemistry 2015 |