 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis of polyfunctional secondary amines by the addition of functionalized zinc reagents to nitrosoarenes†
Vasudevan
Dhayalan
,
Christoph
Sämann
and
Paul
Knochel
*
Department of Chemistry, Ludwig-Maximilians-Universität München, Butenandtstr. 5-13, 81377 Munich, Germany. E-mail: paul.knochel@cup.uni-muenchen.de; Fax: +49-(0)89-2180-77680; Tel: +49-(0)89-2180-77679
First published on 9th January 2015
Abstract
Addition of functionalized aryl, heteroaryl or adamantyl zinc reagents to various nitroso-arenes in the presence of magnesium salts and LiCl in THF produces after a reductive work-up with FeCl2 and NaBH4 in ethanol the corresponding polyfunctional secondary amines in high yields.
The preparation of arylamines is an important synthetic goal since these compounds often have useful properties for pharmaceuticals or material science applications.1 Transition metal catalyzed aminations have been well studied,2 but the use of expensive and toxic metallic catalysts reduces somewhat the utility of such synthetic methods. Another approach has been the use of electrophilic nitrogen reagents and their reactions with non-expensive and low toxic main-group organometallics.3 A few years ago, we have reported that functionalized arylmagnesium reagents add to nitroso-arenes4 and nitro-arenes.5 Although satisfactory yields were obtained, the high reactivity of the carbon–magnesium bond reduces the functional group tolerance. Furthermore, nitroso-arenes have proven to be versatile reagents for performing nitroso aldol and related reactions.6
Herein, we wish to report a mild synthesis of diaryl or heteroaryl(aryl)amines as well as functionalized highly sterically hindered adamantyl(aryl)amines. Thus, the treatment of an arylzinc derivative 2, prepared either by the direct insertion of Mg in the presence of LiCl and ZnCl2 (ref. 7) or by a I/Mg-exchange with iPrMgCl·LiCl8 followed by transmetalation with ZnCl2, with various nitroso-arenes of type 39 affords an intermediate zincated hydroxylamine derivative 4 which after reductive work-up with FeCl2 and NaBH4 in ethanol (25 °C, 15 h) produce the corresponding secondary amines of type 5 in excellent yields (Scheme 1). A range of functional groups have been tolerated in the starting arylzinc reagent as shown in Table 1.
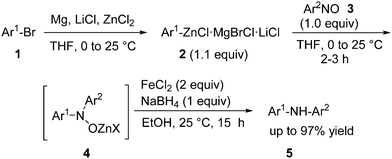 | ||
| Scheme 1 Synthesis of polyfunctional secondary amines of type 5via the addition of functionalized zinc reagents of type 2 to various nitroso compounds of type 3. | ||
| Entry | Zn-reagent | Product, yielda,b (%) |
|---|---|---|
| a General reaction conditions: arylzinc reagent (1.1 equiv.), nitroso electrophile (1.0 equiv.), NaBH4 (1.0 equiv.), FeCl2 (2.0 equiv.). b Yield of analytically pure isolated product as determined by 1H-NMR analysis. c Prepared by I/Mg-exchange with iPrMgCl·LiCl.8 d The TMS-group was cleaved during workup and column chromatography purification. e The arylzinc reagents (2i and 2j) were prepared from the corresponding bromides (see ESI). f Obtained after removal of the ethylene glycol group with CF3CO2H in CH2Cl2 (see ESI). | ||
| 1 | 2a, PhZnCl |

|
| 2 |
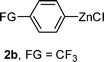
|

|
| 3 | 2c, FG = CO2Etc | 5c: 76 |
| 4 | 2d, FG = tBu | 5d: 96 |
| 5 | 2e, FG = SCH3 | 5e: 70 |
| 6 | 2f, FG = OCH3 | 5f: 78 |
| 7 | 2g, FG = OTMS |

|
| 8 |

|

|
| 9 |

|

|
| 10 |

|

|
Thus, PhZnCl (1.1 equiv.) prepared by the direct insertion of Mg in the presence of LiCl and ZnCl2 reacts with nitrosobenzene 3a (1.0 equiv.) at 25 °C within 2–3 h and produces after reductive work-up with FeCl2 (2.0 equiv.) and NaBH4 (1.0 equiv.) in ethanol (25 °C, 15 h) the corresponding diphenylamine 5a in 85% yield (Table 1, entry 1).10a The presence of both Mg salts and LiCl were found to be essential for achieving a high yield. A variety of arylzinc reagents prepared similarly were used in the addition to 3a. Both electron withdrawing and donating groups can be attached at the aryl ring (Table 1, entries 2–8).10b–g Arylzinc reagent 2c has been prepared via an I/Mg-exchange,8 its reaction with nitrosobenzene (3a) furnishes the corresponding secondary amine 5c in 76% yield (Table 1, entry 3). Although sensitive functional groups like a formyl or an acetyl group are not tolerated, the corresponding bromoacetal (1i) or bromoketal (1j) are readily converted to the zinc reagents (2i and 2j) by the insertion of Mg in the presence of LiCl and ZnCl2.7 The addition of nitrosobenzene (3a) provides after removal of the ethylene glycol protecting group (CF3CO2H in CH2Cl2 at 25 °C for 5–8 h) the secondary amines (5i and 5j) in 64–75% yield (Table 1, entries 9 and 10).
This addition reaction can be extended to various nitroso-arenes (commercially available) or prepared according to the method of Bäckvall.11 Again, electron-donating or accepting substituents are tolerated in the arylnitroso reagents of type 3 furnishing the corresponding diarylamines 5k–r in 77–97% yield (Table 2, entries 1–8).5a,10h–k Noteworthy, a heterocyclic zinc reagent (2m) has also been used as well as a nitrosopyridine (3g)12 leading to heteroaryl(aryl)-amines 5s–y in 55–96% yield (Table 2, entries 9–15). Moreover, tertiary alkylzinc reagents such as t-BuZnCl (6a) and adamantylzinc chloride (6b)13 add to various nitroso-arenes under similar reaction conditions producing otherwise difficult to prepare tertiaryalkyl(aryl)amines 7a–d in 50–89% yield (Table 3, entries 1–4).14
| Entry | Zn-reagent (Ar1) | Electrophile (Ar2) | Product | Yielda,b (%) |
|---|---|---|---|---|
| a General reaction conditions: arylzinc reagent (1.1 equiv.), nitroso electrophile (1.0 equiv.), NaBH4 (1.0 equiv.), FeCl2 (2.0 equiv.). b Yield of analytically pure isolated product as determined by 1H-NMR analysis. c The TMS-group was cleaved during the workup and column chromatography purification. d Prepared by I/Mg-exchange with iPrMgCl·LiCl.8 | ||||
| 1 | 2a |

|
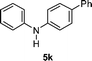
|
79 |
| 2 | 2a |
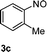
|

|
77 |
| 3 | 2a |
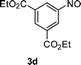
|

|
83 |
| 4 |

|

|

|
97 |
| 5 |

|
3b |

|
90 |
| 6 |

|
3e |

|
81 |
| 7 |
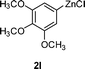
|
3e |

|
97 |
| 8 |

|
3e |

|
97c |
| 9 |

|
3a |

|
63 |
| 10 | 2m | 3d |

|
55 |
| 11 | 2m |
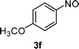
|
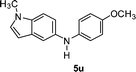
|
60 |
| 12 | 2a |

|

|
67 |
| 13 | 2f | 3g |

|
96 |
| 14 | 2c | 3g |

|
83 |
| 15 | 2m | 3g |

|
70 |
| Entry | Zn-reagent | Electrophile | Product, yielda,b (%) |
|---|---|---|---|
| a General reaction conditions: alkylzinc reagent (1.1 equiv.), nitroso electrophile (1.0 equiv.), NaBH4 (1.0 equiv.), FeCl2 (2.0 equiv.). b Yield of analytically pure isolated product as determined by 1H-NMR analysis. c Prepared by transmetalation of commercially available t-BuMgCl with ZnCl2. | |||
| 1 |
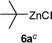
|
3a |
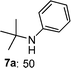
|
| 2 |

|
3a |
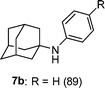
|
| 3 | 6b |
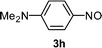
|
7c: R = NMe2 (71) |
| 4 | 6b | 3f | 7d: R = OMe (56) |
In summary, we have shown that aryl, heteroaryl or adamantyl zinc reagents add to various nitroso-arenes in the presence of Mg-salts and LiCl. Both Mg and Li salts are necessary to achieve high yields for the synthesis of the corresponding functionalized secondary amines. Further extensions of this work are currently underway in our laboratories.
The research leading to these results has received funding from the European Research Council under the European Community's Seventh Framework Programme (FP7/2007–2014) ERC grant agreement no. 227763. We thank the Fonds der Chemischen Industrie for financial support. We also thank Heraeus Holding GmbH (Hanau), Rockwood Lithium (Frankfurt), and BASF SE (Ludwigshafen) for the generous gift of chemicals.
Notes and references
- (a) G. Thomas, Medicinal Chemistry, Wiley-VCH, Weinheim, 2000 Search PubMed; (b) S. Liu, X. Jiang, H. Ma, M. S. Liu and A. K.-Y. Jen, Macromolecules, 2000, 33, 3514 CrossRef CAS; (c) W.-L. Yu, J. Pei, W. Huang and A. J. Heeger, Chem. Commun., 2000, 681 RSC; (d) E. Bellmann, S. E. Shaheen, S. Thayumanavan, S. Barlow, R. H. Grubbs, S. R. Marder, B. Kippelen and N. Peyghambarian, Chem. Mater., 1998, 10, 1668 CrossRef CAS; (e) H. Inada, Y. Yonemoto, T. Wakimoto, K. Imai and Y. Shirota, Mol. Cryst. Liq. Cryst., 1996, 280, 331 CrossRef CAS; (f) G. D'Aprano, M. Leclerc, G. Zotti and G. Schiavon, Chem. Mater., 1995, 7, 33 CrossRef.
- (a) M. Wolter, A. Klapars and S. L. Buchwald, Org. Lett., 2001, 3, 3803 CrossRef CAS PubMed; (b) A. Klapars, J. C. Antilla, X. Huang and S. L. Buchwald, J. Am. Chem. Soc., 2001, 123, 7727 CrossRef CAS; (c) C. Desmarets, R. Schneider and Y. Fort, Tetrahedron Lett., 2001, 42, 247 CrossRef CAS; (d) B. H. Lipshutz and H. Ueda, Angew. Chem., Int. Ed., 2000, 39, 4492 CrossRef CAS; (e) R. Shen and J. A. Porco, Jr, Org. Lett., 2000, 2, 1333 CrossRef CAS; (f) A. V. Kalinin, J. F. Bower, P. Riebel and V. Snieckus, J. Org. Chem., 1999, 64, 2986 CrossRef CAS; (g) B. H. Yang and S. L. Buchwald, J. Organomet. Chem., 1999, 576, 125 CrossRef CAS; (h) J. P. Wolfe, S. Wagaw, J.-F. Marcoux and S. L. Buchwald, Acc. Chem. Res., 1998, 31, 805 CrossRef CAS; (i) J. F. Hartwig, Angew. Chem., Int. Ed., 1998, 37, 2046 CrossRef CAS; (j) M. S. Driver and J. F. Hartwig, J. Am. Chem. Soc., 1996, 118, 7217 CrossRef CAS; (k) S. Wagaw and S. L. Buchwald, J. Org. Chem., 1996, 61, 7240 CrossRef CAS; (l) J. P. Wolfe, S. Wagaw and S. L. Buchwald, J. Am. Chem. Soc., 1996, 118, 7215 CrossRef CAS.
- (a) P. Sinha, C. C. Kofink and P. Knochel, Org. Lett., 2006, 8, 3741 CrossRef CAS PubMed; (b) P. Sinha and P. Knochel, Synlett, 2006, 3304 CAS; (c) A. M. Berman and J. S. Johnson, J. Org. Chem., 2006, 71, 219 CrossRef CAS PubMed; (d) A. M. Berman and J. S. Johnson, J. Org. Chem., 2005, 70, 364 CrossRef CAS PubMed; (e) K. Narasaka and M. Kitamura, Eur. J. Org. Chem., 2005, 4505 CrossRef CAS; (f) A. M. Berman and J. S. Johnson, Synlett, 2005, 1799 CAS; (g) A. M. Berman and J. S. Johnson, J. Am. Chem. Soc., 2004, 126, 5680 CrossRef CAS PubMed; (h) M. Kitamura, T. Suga, S. Chiba and K. Narasaka, Org. Lett., 2004, 6, 4619 CrossRef CAS PubMed.
- (a) F. Kopp, I. Sapountzis and P. Knochel, Synlett, 2003, 885 CAS; (b) W. L. Waters and P. G. Marsh, J. Org. Chem., 1975, 40, 3344 CrossRef CAS.
- For the reaction of nitroarenes with Grignard reagents: (a) I. Sapountzis and P. Knochel, J. Am. Chem. Soc., 2002, 124, 9390 CrossRef CAS PubMed; (b) H. Gao, Q. L. Xu, M. Yousufuddin, D. H. Ess and L. Kürti, Angew. Chem., Int. Ed., 2014, 53, 2701 CrossRef CAS PubMed; (c) H. Gao, D. H. Ess, M. Yousufuddin and L. Kürti, J. Am. Chem. Soc., 2013, 135, 7086 CrossRef CAS PubMed; (d) L. Wylie, P. Innocenti, D. K. Whelligan and S. Hoelder, Org. Biomol. Chem., 2012, 10, 4441 RSC; (e) A. Dobbs, J. Org. Chem., 2001, 66, 638 CrossRef CAS; (f) A. P. Dobbs, M. Voyle and N. Whittall, Synlett, 1999, 1594 CrossRef CAS PubMed; (g) M. Bosco, R. Dalpozzo, G. Bartoli, G. Palmieri and M. Petrini, J. Chem. Soc., Perkin Trans. 2, 1991, 657 RSC; (h) L. Barboni, G. Bartoli, E. Marcantoni, M. Petrini and R. Dalpozzo, J. Chem. Soc., Perkin Trans. 1, 1990, 2133 RSC; (i) G. Bartoli, M. Petrini, E. Marcantoni, M. Bosco and R. Dalpozzo, Tetrahedron Lett., 1990, 31, 6089 CrossRef CAS; (j) Y. Inagaki, R. Okazaki and N. Inamoto, Bull. Chem. Soc. Jpn., 1975, 48, 3727 CrossRef CAS; (k) Y. Yost, H. R. Gutmann and C. C. Muscoplat, J. Chem. Soc. C, 1971, 2119 RSC; (l) G. Bartoli, G. Palmieri, M. Bosco and R. Dalpozzo, Tetrahedron Lett., 1989, 30, 2129 CrossRef CAS; (m) D. Y. Curtin and J. C. Kauer, J. Am. Chem. Soc., 1953, 75, 6041 CrossRef CAS.
- (a) A. Yanagisawa, S. Takeshita, Y. Izumi and K. Yoshida, J. Am. Chem. Soc., 2010, 132, 5328 CrossRef CAS PubMed; (b) G.-Q. Tian, J. Yang and K. R. Perez, Org. Lett., 2010, 12, 5072 CrossRef CAS PubMed; (c) M. Lu, D. Zhu, Y. Lu, X. Zeng, B. Tan, Z. Xu and G. Zhong, J. Am. Chem. Soc., 2009, 131, 4562 CrossRef CAS PubMed; (d) H. Yamamoto and M. Kawasaki, Bull. Chem. Soc. Jpn., 2007, 80, 595 CrossRef CAS; (e) H. Yamamoto and N. Momiyama, Chem. Commun., 2005, 3514 RSC; (f) N. Momiyama and H. Yamamoto, J. Am. Chem. Soc., 2005, 127, 1080 CrossRef CAS PubMed; (g) J. M. Janey, Angew. Chem., Int. Ed., 2005, 44, 4292 CrossRef CAS PubMed; (h) P. Merino and T. Tejero, Angew. Chem., Int. Ed., 2004, 43, 2995 CrossRef CAS PubMed; (i) A. Bøgevig, H. Sundén and A. Córdova, Angew. Chem., Int. Ed., 2004, 43, 1109 CrossRef PubMed; (j) Y. Hayashi, J. Yamaguchi, T. Sumiya and M. Shoji, Angew. Chem., Int. Ed., 2004, 43, 1112 CrossRef CAS PubMed; (k) S. P. Brown, M. P. Brochu, C. J. Sinz and D. W. C. MacMillan, J. Am. Chem. Soc., 2003, 125, 10808 CrossRef CAS PubMed; (l) G. Zhong, Angew. Chem., Int. Ed., 2003, 42, 4247 CrossRef CAS PubMed; (m) N. Momiyama and H. Yamamoto, J. Am. Chem. Soc., 2003, 125, 6038 CrossRef CAS PubMed; (n) N. Momiyama and H. Yamamoto, Org. Lett., 2002, 4, 3579 CrossRef CAS PubMed; (o) N. Momiyama and H. Yamamoto, Angew. Chem., Int. Ed., 2002, 41, 2986 CrossRef CAS.
- (a) F. M. Piller, P. Appukkuttan, A. Gavryushin, M. Helm and P. Knochel, Angew. Chem., Int. Ed., 2008, 47, 6802 CrossRef CAS PubMed; (b) F. M. Piller, A. Metzger, M. A. Schade, B. A. Haag, A. Gavryushin and P. Knochel, Chem. – Eur. J., 2009, 15, 7192 CrossRef CAS PubMed.
- (a) A. E. Jensen, W. Dohle, I. Sapountzis, D. M. Lindsay, V. A. Vu and P. Knochel, Synthesis, 2002, 565 CrossRef CAS; (b) I. Sapountzis, W. Dohle and P. Knochel, Chem. Commun., 2001, 2068 RSC; (c) M. Rottländer, L. Boymond, L. Bérillon, A. Leprêtre, G. Varchi, S. Avolio, H. Laaziri, G. Quéguiner, A. Ricci, G. Cahiez and P. Knochel, Chem. – Eur. J., 2000, 6, 767 CrossRef; (d) M. Abarbri, J. Thibonnet, L. Bérillon, F. Dehmel, M. Rottländer and P. Knochel, J. Org. Chem., 2000, 65, 4618 CrossRef CAS PubMed; (e) L. Boymond, M. Rottländer, G. Cahiez and P. Knochel, Angew. Chem., Int. Ed., 1998, 37, 1701 CrossRef CAS.
- (a) O. A. Blackburn, B. J. Coe and M. Helliwell, Organometallics, 2011, 30, 4910 CrossRef CAS; (b) B. Priewisch and K. R. Braun, J. Org. Chem., 2005, 70, 2350 CrossRef CAS PubMed; (c) A. Defoin, Synthesis, 2004, 706 CrossRef CAS PubMed; (d) S. Wang, X. Wang, L. Li and R. C. Advincula, J. Org. Chem., 2004, 69, 9073 CrossRef CAS PubMed; (e) Z. Zhu and J. H. Espenson, J. Org. Chem., 1995, 60, 1326 CrossRef CAS; (f) S. Sakaue, T. Tsubakino, Y. Nishiyama and Y. Ishii, J. Org. Chem., 1993, 58, 3633 CrossRef CAS; (g) E. R. Møller and K. A. Jørgensen, J. Am. Chem. Soc., 1993, 115, 11814 CrossRef; (h) S. T. Tollari, M. Cuscela and F. Porta, J. Chem. Soc., Chem. Commun., 1993, 1510 RSC; (i) W. A. Lees and A. Burawoy, Tetrahedron, 1963, 19, 419 CrossRef CAS.
- (a) N. G. Gaylord, J. Org. Chem., 1960, 25, 1874 CrossRef CAS; (b) Y. Yu, J. Srogl and L. S. Liebeskind, Org. Lett., 2004, 6, 2631 CrossRef CAS PubMed; (c) L. Ackermann, R. Sandmann and W. Song, Org. Lett., 2011, 13, 1784 CrossRef CAS PubMed; (d) D. Craig, J. Am. Chem. Soc., 1935, 57, 195 CrossRef CAS; (e) H. Takeuchi, S. Hirayama, M. Mitani and K. Koyama, J. Chem. Soc., Perkin Trans. 1, 1988, 521 RSC; (f) M. A. Carroll and R. A. Wood, Tetrahedron, 2007, 63, 11349 CrossRef CAS PubMed; (g) D. Maiti and S. L. Buchwald, J. Am. Chem. Soc., 2009, 131, 17423 CrossRef CAS PubMed; (h) J. Piccard, J. Am. Chem. Soc., 1926, 48, 2878 CrossRef CAS; (i) J. Li, M. Cui, A. Yu and Y. Wu, J. Organomet. Chem., 2007, 692, 3732 CrossRef CAS PubMed; (j) R. E. Tundel, K. W. Anderson and S. L. Buchwald, J. Org. Chem., 2006, 71, 430 CrossRef CAS PubMed; (k) M. Kim and S. Chang, Org. Lett., 2010, 12, 1640 CrossRef CAS PubMed.
- D. Zhao, M. Johansson and J.-E. Bäckvall, Eur. J. Org. Chem., 2007, 4431 CrossRef CAS.
- (a) W. Lin, A. Gupta, K. H. Kim, D. Mendel and M. J. Miller, Org. Lett., 2009, 11, 449 CrossRef CAS PubMed; (b) E. C. Taylor, C.-P. Tseng and J. B. Rampal, J. Org. Chem., 1982, 47, 552 CrossRef CAS.
- C. Sämann, V. Dhayalan, P. R. Schreiner and P. Knochel, Org. Lett., 2014, 16, 2418 CrossRef PubMed.
- (a) T. D. Quach and R. A. Batey, Org. Lett., 2003, 5, 4397 CrossRef CAS PubMed; (b) T. Arnauld, D. H. R. Barton and E. Doris, Tetrahedron, 1997, 53, 4137 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures and spectroscopic data for all compounds. See DOI: 10.1039/c4cc08846h |
| This journal is © The Royal Society of Chemistry 2015 |

