Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Impact of a rigid backbone on the structure of an agostically-stabilised dialkylstannylene: isolation of an unusual bridged stannyl–stannylene†
Keith
Izod
*,
Casey M.
Dixon
,
Ross W.
Harrington
and
Michael R.
Probert
Main Group Chemistry Laboratories, School of Chemistry, Newcastle University, Newcastle upon Tyne NE1 7RU, UK. E-mail: keith.izod@ncl.ac.uk; Fax: +44 (0)191 208 6929; Tel: +44 (0)191 208 7101
First published on 21st November 2014
Abstract
The reaction between the phosphine–borane-stabilised dicarbanion complex [1,2-C6H4{CHP(BH3)Cy2}2][Li(THF)n]2 and Cp2Sn gives the unusual stannyl–stannylene [[1,2-C6H4{CHP(BH3)Cy2}2]Sn]2·1½PhMe, in which one dicarbanion ligand chelates a tin centre, while the other bridges a tin–tin bond. The stannylene centre is stabilised by an agostic-type B–H⋯Sn interaction.
In the absence of sufficiently sterically demanding substituents, diorganostannylenes, R2Sn, typically oligomerise to the corresponding polystannanes, –(R2Sn)n– [R = e.g. Et, Cy].1 With more hindered substituents, either distannenes R2Sn
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) SnR2 (I),2 or monomeric stannylenes R2Sn (II) are isolated (Scheme 1);3 for certain substituents a dynamic equilibrium between the distannene and stannylene forms of these compounds has been observed in solution.2b Recently, Power and co-workers reported the terphenyl-substituted mixed valence stannyl–stannylenes ArR2Sn–SnAr (III) [Ar = e.g. 2,6-(2,6-iPr2C6H3)2C6H3; R = H, Me, Ph],4 while both Power and co-workers and Kira and co-workers have isolated a small number of bridged analogues.5 Such stannyl–stannylene compounds are formal isomers of distannenes, in which a tetravalent (formally Sn(III)) and a divalent (formally Sn(I)) centre are connected by a σ-bond. Calculations on III (R = H) suggest that this isomer is the global minimum on the potential energy surface, although the corresponding distannene (I) and hydride-bridged isomers ArSn(μ-H)2SnAr (IV) are less stable by only 7–14 kcal mol−1.4d
SnR2 (I),2 or monomeric stannylenes R2Sn (II) are isolated (Scheme 1);3 for certain substituents a dynamic equilibrium between the distannene and stannylene forms of these compounds has been observed in solution.2b Recently, Power and co-workers reported the terphenyl-substituted mixed valence stannyl–stannylenes ArR2Sn–SnAr (III) [Ar = e.g. 2,6-(2,6-iPr2C6H3)2C6H3; R = H, Me, Ph],4 while both Power and co-workers and Kira and co-workers have isolated a small number of bridged analogues.5 Such stannyl–stannylene compounds are formal isomers of distannenes, in which a tetravalent (formally Sn(III)) and a divalent (formally Sn(I)) centre are connected by a σ-bond. Calculations on III (R = H) suggest that this isomer is the global minimum on the potential energy surface, although the corresponding distannene (I) and hydride-bridged isomers ArSn(μ-H)2SnAr (IV) are less stable by only 7–14 kcal mol−1.4d
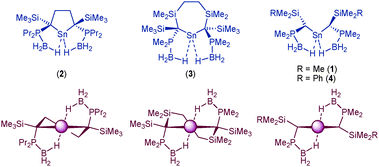
We have recently shown that agostic-type B–H⋯E interactions stabilise monomeric dialkylstannylenes, significantly disfavouring dimerisation to the corresponding distannene.6 For example, [(Me3Si){Me2P(BH3)}C]2Sn (1),6d which exhibits two stabilising B–H⋯Sn contacts, adopts a monomeric structure, whereas, {(Me3Si)2CH}2Sn,2c which is isoelectronic and isosteric with 1, but which lacks such agostic-type interactions, dimerises to the corresponding distannene in the solid state. In all of the dialkylstannylenes we have isolated which exhibit these stabilising contacts (Scheme 2) the supporting ligand is either monodentate (1, 4), or else has a flexible spacer group linking the two “carbanion” centres (2, 3). This permits the phosphine–borane group to tilt towards the tin centre, maximising overlap between the B–H σ-orbital and the vacant p-orbital at tin and so providing the greatest possible stabilisation of the electron-poor tin(II) centre.
Recently we have begun to explore the chemistry of phosphine–borane-stabilised dicarbanions in which the two carbanion centres are linked by an ortho-phenylene spacer.7 We were interested to explore the impact of such a rigid ligand backbone on the strength of the B–H⋯Sn agostic-type interactions in the corresponding dialkylstannylene derivatives, since the rigidity of the ligand should limit the degree to which the borane group can position itself close to the tin atom and so moderate the strength of the stabilising B–H⋯Sn interactions. We report herein our initial studies in this area and the unexpected isolation of an agostically-stabilised, bridged stannyl–stannylene.
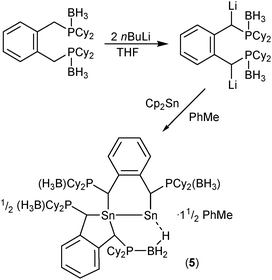
The reaction between in situ-generated [1,2-C6H4{CHP(BH3)Cy2}2][Li(THF)n]27 and one equivalent of Cp2Sn8 in toluene gives a yellow solution containing pale solids of CpLi (Scheme 3). Removal of these solids by filtration, followed by concentration and cooling of the filtrate, yields pale yellow crystals of [[1,2-C6H4{CHP(BH3)Cy2}2]Sn]2·1½PhMe (5) after 1 week.
Somewhat surprisingly, once isolated in the solid state, 5 has limited solubility in aromatic and ethereal solvents and reacts rapidly with chlorinated solvents. Compound 5 is also air-sensitive, decomposes on exposure to ambient light or temperatures above ca. 50 °C, and, over a period of several hours, begins to decompose in THF solution at room temperature. Nonetheless, 5 is sufficiently soluble and stable in THF for limited characterisation by NMR spectroscopy (see below). Single crystals of 5 suitable for X-ray crystallography were obtained from a freshly-prepared sample in toluene.
Compound 5 crystallises as a discrete molecular species containing two distinct tin centres joined by a formal Sn–Sn σ-bond and with 1½ molecules of toluene in the asymmetric unit (Fig. 1).‡ The formal Sn(III) centre is bonded to the two carbon atoms of a chelating dicarbanion ligand [Sn(1)–C(1) 2.1887(17), Sn(1)–C(8) 2.2021(18) Å] and to a single carbon atom of the second dicarbanion ligand [Sn(1)–C(33) 2.2518(17) Å], along with the short Sn–Sn contact. In contrast, the formal Sn(I) centre is bonded to the adjacent tin atom and a carbon atom of the second dicarbanion ligand [Sn(2)–C(40) 2.3250(18) Å]. In addition, Sn(2) has a short contact to a hydrogen atom of one of the BH3 groups of the ligand which chelates Sn(1) [Sn(2)⋯H(2B) 2.49(2) Å]. Although the location of H atoms by X-ray crystallography is rather imprecise, this distance is substantially shorter than the sum of the van der Waals radii of Sn and H (3.37 Å), suggesting that there is a significant, agostic-type B–H⋯Sn interaction. This distance is similar to the H⋯Sn distances in 1–4, which fall in the range 2.03(5)–2.41(8) Å.6
Thus, one phosphine–borane-stabilised carbanion ligand chelates Sn(1), generating a five-membered C4Sn heterocycle, while the second bridges Sn(1) and Sn(2), generating a C4Sn2 six-membered heterocycle; each of these ligands adopts a meso-configuration. The Sn(1)–Sn(2) distance [2.81531(17) Å] is substantially shorter than the Sn–Sn distances in the few previously reported stannyl–stannylenes, which range from 2.865 to 2.9688(5) Å,3,9 and is similar to the Sn–Sn distance in grey tin (2.80 Å).10 We attribute the shortness of this distance to the incorporation of the Sn–Sn bond into a rigid six-membered ring.
DFT calculations on the model complex [[1,2-C6H4{CHP(BH3)Me2}2]Sn]2 (5′), in which the cyclohexyl groups have been replaced by smaller methyl substituents, reproduce well the core structure of 5 observed in the solid state (Fig. 2). In particular, one of the borane hydrogen atoms lies close to the divalent Sn centre (Sn⋯H 2.13 Å). Natural Bond Orbital (NBO) analysis indicates that this interaction stabilises 5′ by 28.8 kcal mol−1, similar to the stabilisation energies calculated for 1–4 (in spite of the unusual bridging mode of the ligand in 5′).
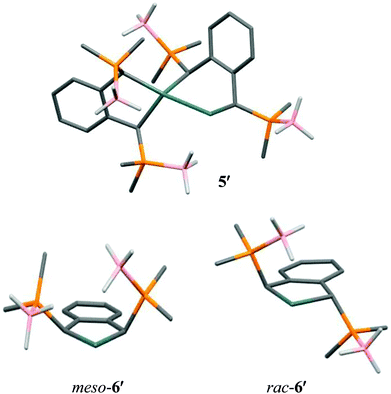 | ||
| Fig. 2 Optimised geometries of 5′, meso-6′ and rac-6′ with C-bound H atoms omitted for clarity [B3LYP/6-31G(2d,p)[LanL2DZ on Sn]] (C dark grey, H white, B pink, P orange, Sn light grey). | ||
In order to estimate the stability of the stannyl–stannylene 5′ in comparison to the putative monomer meso-[1,2-C6H4{CHP(BH3)Me2}2]Sn (meso-6′) we have calculated the energy of both this species and the corresponding rac isomer (Fig. 2). The optimised geometries for both meso- and rac-6′ exhibit a single short B–H⋯Sn contact with Sn⋯H distances of 2.15 and 2.13 Å, respectively. While for meso-6′ this interaction leaves the C4Sn five-membered ring essentially planar, for rac-6′ it results in a twisting of both the five-membered ring and the aromatic ring, such that the C–Sn–C plane lies at approximately 8° to the mean plane of the aromatic backbone. NBO analysis suggests that the B–H⋯Sn interactions stabilise meso- and rac-6′ by 41.4 and 33.1 kcal mol−1, respectively. Nonetheless, rac-6′ is calculated to be 1.8 kcal mol−1 more stable than meso-6′. Comparison of the energy of meso-6′ with that of the stannyl–stannylene 5′ suggests that the latter is favoured by just 1.1 kcal mol−1.
A 31P{1H} NMR spectrum of the crude reaction solution, prior to the isolation of 5, consists of multiple broad and overlapping signals. This suggests that, in addition to 5, the solution contains the corresponding rac isomer, either as a monomeric or dimeric species;§ however, we have, as yet, been unable to isolate this species. The 1H NMR spectrum of 5 is complex and rather uninformative, due to the overlap of signals from the eight chemically inequivalent cyclohexyl groups (within which pairs of CH2 groups are diastereotopic) and the signals from the four chemically inequivalent benzylic and borane groups. However, the 31P{1H} NMR spectrum of 5 exhibits four broad, approximately equal intensity signals at 27.2, 28.6, 30.0, and 35.4 ppm, while the 11B{1H} NMR spectrum exhibits two broad signals at −43.5 and −38.8 ppm in an approximately 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio; in the latter case, we assign the unique high field signal to the phosphine–borane group associated with the short B–H⋯Sn interaction. These spectra indicate that the dinuclear structure of 5 observed in the solid state persists in solution. Consistent with this, the 119Sn{1H} NMR spectrum of 5 exhibits broad, featureless signals at −103 and 339 ppm, which we attribute to the stannyl and stannylene centres, respectively; coupling between the two Sn centres is not resolved, due to the broad nature of these signals. The former signal is typical of tetravalent tin centres, while the latter is similar to the chemical shifts of the previously reported agostically-stabilised dialkylstannylenes 1–4, which fall into the range 320–787 ppm.6
3 ratio; in the latter case, we assign the unique high field signal to the phosphine–borane group associated with the short B–H⋯Sn interaction. These spectra indicate that the dinuclear structure of 5 observed in the solid state persists in solution. Consistent with this, the 119Sn{1H} NMR spectrum of 5 exhibits broad, featureless signals at −103 and 339 ppm, which we attribute to the stannyl and stannylene centres, respectively; coupling between the two Sn centres is not resolved, due to the broad nature of these signals. The former signal is typical of tetravalent tin centres, while the latter is similar to the chemical shifts of the previously reported agostically-stabilised dialkylstannylenes 1–4, which fall into the range 320–787 ppm.6
In summary, while phosphine–borane-stabilised dicarbanions linked by a flexible aliphatic spacer group give the corresponding stannylene derivatives, the incorporation of a rigid ortho-phenylene spacer leads to the isolation of the stannyl–stannylene complex 5. Nonetheless, 5 exhibits an agostic-type B–H⋯Sn interaction which stabilises this complex by 28.8 kcal mol−1.
The authors would like to acknowledge the use of the EPSRC UK National Service for Computational Chemistry Software (NSCCS) at Imperial College London in carrying out this work.
Notes and references
- A. G. Davies, in Comprehensive Organometallic Chemistry III, ed. D. M. P. Mingos, R. H. Crabtree and C. E. Housecroft, Elsevier, Oxford, UK, 2007, ch. 3.14, vol. 3 Search PubMed.
- (a) P. J. Davidson and M. F. Lappert, J. Chem. Soc., Chem. Commun., 1973, 317 RSC; (b) K. W. Zilm, G. A. Lawless, R. M. Merrill, J. M. Millar and G. G. Webb, J. Am. Chem. Soc., 1987, 109, 7236 CrossRef CAS; (c) D. E. Goldberg, D. H. Harris, M. F. Lappert and K. M. Thomas, J. Chem. Soc., Chem. Commun., 1976, 261 RSC; (d) M. Sturmann, W. Saak, K. W. Klinkhammer and M. Weidenbruch, Z. Anorg. Allg. Chem., 1999, 625, 1955 CrossRef CAS; (e) T. Fukawa, V. Y. Lee, M. Nakamoto and A. Sekiguchi, J. Am. Chem. Soc., 2004, 126, 11758 CrossRef CAS PubMed; (f) D. Kurzbach, S. Yao, D. Hindenberger and K. W. Klinkhammer, Dalton Trans., 2010, 39, 6449 RSC; (g) V. Y. Lee, T. Fukawa, M. Nakamoto, A. Sekiguchi, B. L. Tumanskii, M. Karni and Y. Apeloig, J. Am. Chem. Soc., 2006, 128, 11643 CrossRef CAS PubMed; (h) J. Henning and L. Wesemann, Angew. Chem., Int. Ed., 2012, 51, 12869 CrossRef CAS PubMed.
- For examples see: (a) P. Jutzi, A. Becker, H. G. Stammler and B. Neumann, Organometallics, 1991, 10, 1647 CrossRef CAS; (b) M. Kira, R. Yauchibara, R. Hirano, C. Kabuto and H. Sakurai, J. Am. Chem. Soc., 1991, 113, 7785 CrossRef CAS; (c) M. Kira, S. Ishida, T. Iwamoto and C. Kabuto, J. Am. Chem. Soc., 1999, 121, 9722 CrossRef CAS; (d) M. Kira, S. Ishida, T. Iwamoto, M. Ichinohe, C. Kabuto, L. Ignatovich and H. Sakurai, Chem. Lett., 1999, 263 CrossRef CAS; (e) C. Eaborn, M. S. Hill, P. B. Hitchcock, D. Patel, J. D. Smith and S. Zhang, Organometallics, 2000, 19, 49 CrossRef CAS; (f) C. Eaborn, T. Ganicz, P. B. Hitchcock, J. D. Smith and S. E. Sozerli, Organometallics, 1997, 16, 5621 CrossRef CAS; (g) A. D. Phillips, S. Hino and P. P. Power, J. Am. Chem. Soc., 2003, 125, 7520 CrossRef CAS PubMed; (h) W. Setaka, K. Sakamoto, M. Kira and P. P. Power, Organometallics, 2001, 20, 4460 CrossRef CAS; (i) B. E. Eichler, B. L. Phillips, P. P. Power and M. P. Augustine, Inorg. Chem., 2000, 39, 5450 CrossRef CAS; (j) H. Grutzmacher, H. Pritzkow and F. T. Edelmann, Organometallics, 1991, 10, 23 CrossRef; (k) S. Wingerter, H. Gornitzka, R. Bertermann, S. K. Pandey, J. Rocha and D. Stalke, Organometallics, 2000, 19, 3890 CrossRef CAS; (l) C. Drost, P. B. Hitchcock, M. F. Lappert and L. J.-M. Pierssens, Chem. Commun., 1997, 1141 RSC; (m) L. Pu, A. D. Phillips, A. F. Richards, M. Stender, R. S. Simons, M. M. Olmstead and P. P. Power, J. Am. Chem. Soc., 2003, 125, 11626 CrossRef CAS PubMed.
- (a) A. D. Phillips, S. Hino and P. P. Power, J. Am. Chem. Soc., 2003, 125, 7520 CrossRef CAS PubMed; (b) Y. Peng, M. Brynda, B. D. Ellis, J. C. Fettinger, E. Rivard and P. P. Power, Chem. Commun., 2008, 6042 RSC; (c) B. E. Eichler and P. P. Power, Inorg. Chem., 2000, 39, 5444 CrossRef CAS; (d) E. Rivard, R. C. Fischer, R. Wolf, Y. Peng, W. A. Merrill, N. D. Schley, Z. Zhu, L. Pu, J. C. Fettinger, S. J. Teat, I. Nowik, R. H. Herber, N. Takagi, S. Nagase and P. P. Power, J. Am. Chem. Soc., 2007, 129, 16197 CrossRef CAS PubMed; (e) H. Lei, J. C. Fettinger and P. P. Power, Organometallics, 2010, 29, 5585 CrossRef CAS; (f) P. P. Power, C. Stanciu, I. Nowik and R. H. Herber, Inorg. Chem., 2005, 44, 9461 CrossRef CAS PubMed.
- (a) W. Setaka, K. Hirai, H. Tomioka, K. Sakamoto and M. Kira, Chem. Commun., 2008, 6558 RSC; (b) S. Hino, M. M. Olmstead and P. P. Power, Organometallics, 2005, 24, 5484 CrossRef CAS; (c) C. Stanciu, A. F. Richards and P. P. Power, J. Am. Chem. Soc., 2004, 126, 4106 CrossRef CAS PubMed.
- (a) K. Izod, W. McFarlane, B. V. Tyson, I. Carr, W. Clegg and R. W. Harrington, Organometallics, 2006, 25, 1135 CrossRef CAS; (b) K. Izod, W. McFarlane, C. Wills, W. Clegg and R. W. Harrington, Organometallics, 2008, 27, 4386 CrossRef CAS; (c) K. Izod, C. Wills, W. Clegg and R. W. Harrington, Organometallics, 2009, 28, 2211 CrossRef CAS; (d) K. Izod, C. Wills, W. Clegg and R. W. Harrington, Organometallics, 2009, 28, 5661 CrossRef CAS; (e) K. Izod, C. Wills, W. Clegg and R. W. Harrington, J. Organomet. Chem., 2013, 725, 11 CrossRef CAS PubMed.
- K. Izod, C. M. Dixon, E. McMeekin, L. Rodgers, R. W. Harrington and U. Baisch, Organometallics, 2014, 33, 378 CrossRef CAS.
- E. O. Fischer and H. Grubert, Z. Naturforsch., B: J. Chem. Sci., 1956, 11, 423 Search PubMed.
- (a) B. E. Eichler, A. D. Phillips and P. P. Power, Organometallics, 2003, 22, 5423 CrossRef CAS; (b) C. J. Cardin, D. J. Cardin, S. P. Constantine, S. J. Teat and S. Coles, Organometallics, 1998, 17, 2144 CrossRef CAS; (c) C. Drost, M. Hildebrand and P. Lönnecke, Main Group Met. Chem., 2002, 25, 93 CrossRef CAS PubMed.
- N. N. Greenwood and A. Earnshaw, Chemistry of the Elements, Butterworth-Heinemann, Oxford, UK, 2nd edn, 1997 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Details of the synthesis of 5; details of the DFT studies of 5′, rac-6′, and meso-6′. CCDC 1010284. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4cc08740b |
| ‡ Crystal data for 5: C64H112B4P4Sn2·1.5C7H8, FW = 1424.36, monoclinic, P21/c, a = 14.0885(1), b = 26.5770(2), c = 20.0509(2) Å, β = 95.2730(10)°, V = 7475.89(11) Å3, Z = 2, μ = 0.793 mm−1, final R1 = 0.028 for I > 2σI, wR2 = 0.060 for all data, GoF = 1.051. CCDC 1010284. |
| § The relatively low isolated yield of 5 (28%) is a reflection of the mixture of isomers produced in this reaction; if an equal ratio of rac to meso isomers is assumed, then this equates to an isolated yield of 56% of the total meso diastereomer. |
| This journal is © The Royal Society of Chemistry 2015 |