Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Dual-responsive nanoparticles that aggregate under the simultaneous action of light and CO2†
Ji-Woong
Lee
and
Rafal
Klajn
*
Department of Organic Chemistry, Weizmann Institute of Science, 76100 Rehovot, Israel. E-mail: rafal.klajn@weizmann.ac.il
First published on 18th November 2014
Abstract
Metallic nanoparticles co-functionalised with monolayers of UV- and CO2-sensitive ligands were prepared and shown to respond to these two types of stimuli reversibly and in an orthogonal fashion. The composition of the coating could be tailored to yield nanoparticles capable of aggregating exclusively when both UV and CO2 were applied at the same time, analogously to the behaviour of an AND logic gate.
Stimuli-responsive nanomaterials1 that self-assemble in a reversible fashion are important because of their emerging applications in diverse fields, such as switchable catalysis,2,3 water purification,4 detection of analytes,5 and time-sensitive information storage.6 Accordingly, a variety of nanoparticles (NPs) have been engineered to aggregate in response to different external stimuli, such as a magnetic field,7 pH,8,9 or chemical fuel.10 For example, dynamic aggregation of metallic NPs was accomplished by reversibly photoswitching the electronic states and geometries of surface-bound chromophores.11–14 New applications could arise, however, for NPs that respond to two (or more) different external stimuli at once, such as the recently reported dual-responsive NPs that aggregate when either a magnetic field or light is applied.15–18 Whereas such dual-responsiveness is, in principle, relatively easy to “encode” in the NPs, it is more challenging to design systems capable of aggregating only when two types of external stimuli are applied at the same time.19,20 Here, we describe the first system exhibiting such behaviour with light and carbon dioxide as the two “inputs”.
Carbon dioxide is a biocompatible, naturally abundant gas that has several advantages over other chemical stimuli: (i) facile reactivity with various nucleophilic Lewis base functional groups such as amines, NHCs (N-heterocyclic carbenes), or guanidines, (ii) easy removal from the above complexes by mild heating, sonication, or purging with an inert gas, (iii) no by-product formation through the reversible complex formation, and (iv) potential applicability to reversible CO2 capture and/or transformations.21–24 In addition, nanomaterials that aggregate reversibly in the presence of CO2 are potentially important as possible anti-cancer therapeutics (taking advantage of the increased production of CO2 in cancer cells25,26). Although many CO2-sensitive materials,27 in particular, organic polymers,28,29 have been reported, the application of amine-functionalised metallic NPs as CO2-responsive materials remains unknown. Here, we presumed that control of the NP surface polarity by using fine-tuned amounts of photo- and CO2-sensitive ligands could induce selective and reversible aggregation of NPs with two orthogonal stimuli (Fig. 1).
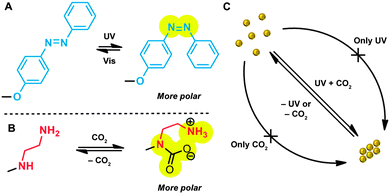 | ||
| Fig. 1 (A) Reversible isomerisation of a photoswitchable ligand. (B) Reversible CO2 complexation by a diamine ligand. (C) A dual-stimuli-responsive AND logic gate. | ||
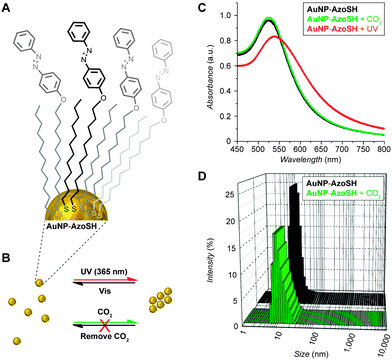
It is well established that mixtures of ω-functionalised long-chain alkanethiols can form mixed self-assembled monolayers (mSAMs) on the surfaces of metallic NPs30 (here, we focused on gold NPs). We commenced our study with AuNPs functionalised with mSAMs comprising an azobenzene-terminated thiol (AzoSH) and a “background” ligand, 1-dodecanethiol (C12SH) (Fig. 2a). As the UV-Vis spectra in Fig. 2c show, NPs decorated with critical (>50%) amounts of AzoSH aggregated in toluene upon exposure to long-wave (λ = 365 nm) UV light,31 whereas they exhibited no detectable response to CO2 (confirmed by dynamic light scattering (DLS) measurements; see ESI,† Fig. S2 in Section S5). Furthermore, we observed that the trans-to-cis photoisomerisation of AzoSH did not affect the affinity of these NPs towards CO2 (Fig. S2, ESI†). Therefore, we concluded that those AuNPs bearing only the AzoSH and C12SH ligands are inert towards the CO2 stimulus under these conditions.
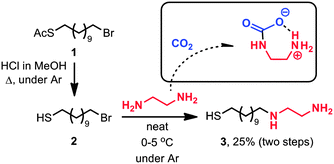
The lack of general knowledge on the construction of CO2-sensitive metal-nanoparticle surfaces encouraged us to investigate the synthesis and application of thiol-terminated amine ligands (Fig. 1b).32 Based on previous literature reports,33–36 we presumed that ethylenediamine derivatives are capable of CO2 complexation under low-pressure conditions. To verify the effect of CO2 binding on the solubility in toluene, we synthesised and studied the behaviour of a model compound, N-decylethylenediamine. As detailed in the ESI† (Section S3), this diamine was readily soluble in toluene; however, rapid precipitation commenced upon bubbling CO2 through the solution (Fig. S1, ESI†). The process was reversible: the addition of an inert gas (e.g. N2) caused the precipitate to redissolve. Encouraged by these results, we then proceeded to synthesise AuNPs decorated with the diamine moieties. Although diamine-functionalised NPs were previously prepared by reacting 11-bromoundecane-1-thiol (2)-decorated NPs with 1,2-ethylenediamine, we began our investigation by preparing a well-defined thiol bearing the diamine moiety. As shown in Scheme 1, the synthesis of diamine 3 was straightforward, starting with a known material 1.37–39
With the diamine-functionalised thiolate ligand 3 in hand, we prepared a family of dual stimuli-responsive AuNPs by varying the ratio of the two responsive ligands (AzoSH and diamine 3) and the background ligand 1-dodecanethiol (C12SH), as shown in Fig. 3. Although 3 could potentially bind to AuNPs via both the mercapto and the amine functionalities, the latter scenario is unlikely given the ca. two-orders-of-magnitude higher affinity of Au to thiols as compared to amines.12 In addition, binding to Au via the amine functionalities would cause NP crosslinking and irreversible aggregation, which we did not observe.40
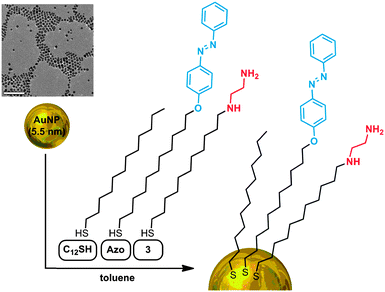 | ||
| Fig. 3 Preparation of dual-responsive AuNPs functionalised with an mSAM comprising C12SH, AzoSH and diamine 3 (the scale bar in the TEM image is 50 nm). | ||
Owing to the high polarity and low toluene solubility of the diamine ligand 3, the ligand exchange reaction was conducted under dilute (ca. 10 times) conditions, compared with typical ligand exchange reaction conditions.41 Moreover, 3 was always used after drying under vacuum at 40 °C to ensure that it was not complexed with CO2. The functionalised AuNPs were precipitated with methanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
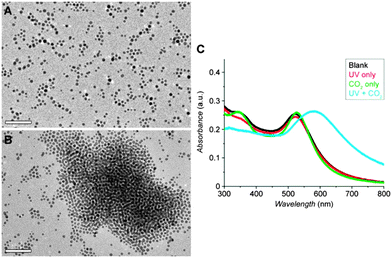
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) in the presence of didodecyldimethylammonium bromide (0.5 mg per 0.716 mg of Au), washed extensively with methanol to remove any unbound molecules, dried under vacuum and kept in an inert atmosphere prior to use. A good indication of the presence of 3 in the functionalised AuNPs is their good solubility in polar solvents (e.g., ethanol, methanol and even water), which confirms a successful ligand–exchange reaction (for comparison, AuNP–AzoSH is soluble only in non-polar solvents such as toluene, chloroform, or dichloromethane). The functionalisation procedure did not affect the size or size distribution of the NPs, whose monodispersity was confirmed by transmission electron microscopy (Fig. 5a).
1 v/v) in the presence of didodecyldimethylammonium bromide (0.5 mg per 0.716 mg of Au), washed extensively with methanol to remove any unbound molecules, dried under vacuum and kept in an inert atmosphere prior to use. A good indication of the presence of 3 in the functionalised AuNPs is their good solubility in polar solvents (e.g., ethanol, methanol and even water), which confirms a successful ligand–exchange reaction (for comparison, AuNP–AzoSH is soluble only in non-polar solvents such as toluene, chloroform, or dichloromethane). The functionalisation procedure did not affect the size or size distribution of the NPs, whose monodispersity was confirmed by transmission electron microscopy (Fig. 5a).
Fine-tuning of the ratio of the three ligands was critical for obtaining NPs exhibiting the behaviour shown in Fig. 1c (in this context, it is worth mentioning that the previously adapted32,34 on-nanoparticle reaction between ethylenediamine and 2-functionalised AuNPs would hardly be applicable). We found that when the molar percentage of 3 in the NPs exceeded 25%, the particles were insoluble in toluene due to their high surface polarity. These aggregated NPs could not be redissolved using visible light, sonication, or by purging with N2 (to remove any possibly complexed CO2). Therefore, the amount of diamine 3 was fixed at 25% throughout the optimisation of the mSAM composition. Having screened the ratio of the remaining two ligands (AzoSH and C12SH) from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 to 2
2 to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
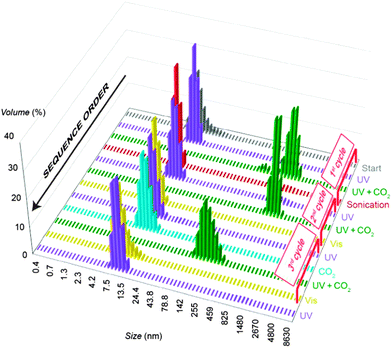
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, we found that NPs containing 33–35 mol% of AzoSH within the mSAM exhibited the behaviour shown in Fig. 1c. According to DLS, these NPs had hydrodynamic diameters of 7–10 nm in toluene, and did not aggregate upon either only UV irradiation or CO2 treatment (bubbling into the solution) (purple and blue bars, respectively, in Fig. 4). Only when both stimuli were applied at once (4 min of UV irradiation during CO2 bubbling) did the NPs rapidly assemble into aggregates of 800–1000 nm in size, as shown by DLS (green bars in Fig. 4). The presence of aggregated NPs was also evident from TEM and spectroscopic analysis (Fig. 5b and c). Interestingly, these aggregates were stable in a closed vial placed in the dark for more than 30 minutes.
1, we found that NPs containing 33–35 mol% of AzoSH within the mSAM exhibited the behaviour shown in Fig. 1c. According to DLS, these NPs had hydrodynamic diameters of 7–10 nm in toluene, and did not aggregate upon either only UV irradiation or CO2 treatment (bubbling into the solution) (purple and blue bars, respectively, in Fig. 4). Only when both stimuli were applied at once (4 min of UV irradiation during CO2 bubbling) did the NPs rapidly assemble into aggregates of 800–1000 nm in size, as shown by DLS (green bars in Fig. 4). The presence of aggregated NPs was also evident from TEM and spectroscopic analysis (Fig. 5b and c). Interestingly, these aggregates were stable in a closed vial placed in the dark for more than 30 minutes.
Further demonstration of the AND gate-like behaviour was provided by removing one of the two stimuli. For example, when a solution of aggregated NPs was sonicated, facile disaggregation was observed even under constant UV irradiation (red bars in Fig. 4; ca. 10 nm). The resulting NPs did not respond to only CO2 or UV, but they could again be quickly aggregated by applying UV and CO2 simultaneously (second-cycle green bars in Fig. 4 and the TEM image in Fig. 5b). Interestingly, our NPs also assembled as a result of CO2 bubbling (4 min), followed by exposure to UV light, or via the opposite sequence, whereby UV-irradiated NPs were treated with CO2 in the dark: these results indicate that both (i) cis-azobenzene and (ii) the CO2–diamine complex are relatively stable, (i) in the dark, and (ii) unless other stimulants (such as sonication, heating or inert gas bubbling) are applied.
Thus assembled AuNPs readily deaggregated under ambient light (5–6 minutes) or upon exposure to visible light (fluorescent bulb, <30 seconds), and consequently, additional assembly–disassembly cycles could be performed. Overall, we concluded that this study represents the first example of CO2- and light-responsive nanoparticles, whose behaviour constitutes an AND logic gate. Noteworthy, NPs having higher surface concentrations of AzoSH did respond to UV light by assembling into aggregates, which could aggregate further upon the delivery of CO2 (ESI,† Fig. S6C). In other words, only upon pre-exposure to UV light were these specific NPs responsive to CO2.
We prepared gold nanoparticles co-functionalised with azobenzene- and diamine-terminated ligands, which rendered the particles responsive to UV irradiation and CO2, respectively. Exposure to UV and CO2 led to a stepwise increase in the nanoparticle surface polarity, triggering aggregation in a non-polar medium (toluene).42 The composition of the mixed monolayer of ligands could be tuned in order to afford NPs capable of aggregating only when both types of stimuli are applied at once. Both of these stimuli could easily be turned off/removed without the generation of any by-products/chemical waste, after which the assembly–disassembly cycles could be repeated. The aggregates exist only when both light and CO2 are applied simultaneously. An important future direction concerning these and other man-made dynamically aggregating materials is to study/engineer their emergent behaviours under non-equilibrium conditions.
Notes and references
- Intelligent Stimuli-Responsive Materials. From Well-Defined Nanostructures to Applications, ed. Q. Li, John Wiley and Sons, Hoboken, 2013 Search PubMed.
- V. Polshettiwar, R. Luque, A. Fihri, H. B. Zhu, M. Bouhrara and J. M. Bassett, Chem. Rev., 2011, 111, 3036–3075 CrossRef CAS PubMed.
- Y. H. Zhu, L. P. Stubbs, F. Ho, R. Z. Liu, C. P. Ship, J. A. Maguire and N. S. Hosmane, ChemCatChem, 2010, 2, 365–374 CrossRef CAS.
- C. T. Yavuz, J. T. Mayo, W. W. Yu, A. Prakash, J. C. Falkner, S. Yean, L. L. Cong, H. J. Shipley, A. Kan, M. Tomson, D. Natelson and V. L. Colvin, Science, 2006, 314, 964–967 CrossRef PubMed.
- R. Elghanian, J. J. Storhoff, R. C. Mucic, R. L. Letsinger and C. A. Mirkin, Science, 1997, 277, 1078–1081 CrossRef CAS.
- R. Klajn, P. J. Wesson, K. J. M. Bishop and B. A. Grzybowski, Angew. Chem., Int. Ed., 2009, 48, 7035–7039 CrossRef CAS PubMed.
- G. Singh, H. Chan, A. Baskin, E. Gelman, N. Repnin, P. Kral and R. Klajn, Science, 2014, 345, 1149–1153 CrossRef CAS PubMed.
- R. Sardar, N. S. Bjorge and J. S. Shumaker-Parry, Macromolecules, 2008, 41, 4347–4352 CrossRef CAS.
- J. Simard, C. Briggs, A. K. Boal and V. M. Rotello, Chem. Commun., 2000, 1943–1944 RSC.
- J. Boekhoven, A. M. Brizard, K. N. K. Kowlgi, G. J. M. Koper, R. Eelkema and J. H. van Esch, Angew. Chem., Int. Ed., 2010, 49, 4825–4828 CrossRef CAS PubMed.
- A. Manna, P. L. Chen, H. Akiyama, T. X. Wei, K. Tamada and W. Knoll, Chem. Mater., 2003, 15, 20–28 CrossRef CAS.
- R. Klajn, K. J. M. Bishop and B. A. Grzybowski, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 10305–10309 CrossRef CAS PubMed.
- A. Kohntopp, A. Dabrowski, M. Malicki and F. Temps, Chem. Commun., 2014, 50, 10105–10107 RSC.
- R. Klajn, J. F. Stoddart and B. A. Grzybowski, Chem. Soc. Rev., 2010, 39, 2203–2237 RSC.
- H. Han, J. Y. Lee and X. M. Lu, Chem. Commun., 2013, 49, 6122–6124 RSC.
- J. H. Schenkel, A. Samanta and B. J. Ravoo, Adv. Mater., 2014, 26, 1076–1080 CrossRef CAS PubMed.
- S. Das, P. Ranjan, P. S. Maiti, G. Singh, G. Leitus and R. Klajn, Adv. Mater., 2013, 25, 422–426 CrossRef CAS PubMed.
- O. Chovnik, R. Balgley, J. R. Goldman and R. Klajn, J. Am. Chem. Soc., 2012, 134, 19564–19567 CrossRef CAS PubMed.
- C. Stoffelen, J. Voskuhl, P. Jonkheijm and J. Huskens, Angew. Chem., Int. Ed., 2014, 53, 3400–3404 CrossRef CAS PubMed.
- D. B. Liu, W. W. Chen, K. Sun, K. Deng, W. Zhang, Z. Wang and X. Y. Jiang, Angew. Chem., Int. Ed., 2011, 50, 4103–4107 CrossRef CAS PubMed.
- C. D. Gomes, O. Jacquet, C. Villiers, P. Thuery, M. Ephritikhine and T. Cantat, Angew. Chem., Int. Ed., 2012, 51, 187–190 CrossRef PubMed.
- M. North, R. Pasquale and C. Young, Green Chem., 2010, 12, 1514–1539 RSC.
- S. N. Riduan, Y. G. Zhang and J. Y. Ying, Angew. Chem., Int. Ed., 2009, 48, 3322–3325 CrossRef CAS PubMed.
- P. G. Jessop, D. J. Heldebrant, X. W. Li, C. A. Eckert and C. L. Liotta, Nature, 2005, 436, 1102 CrossRef CAS PubMed.
- C. U. Vohwinkel, E. Lecuona, H. Y. Sun, N. Sommer, I. Vadasz, N. S. Chandel and J. I. Sznajder, J. Biol. Chem., 2011, 286, 37067–37076 CrossRef CAS PubMed.
- M. Varughese, S. Patole, A. Shama and J. Whitehall, Pediatr. Pulmonol., 2002, 33, 56–64 CrossRef CAS PubMed.
- K. C. Jie, Y. Yao, X. D. Chi and F. H. Huang, Chem. Commun., 2014, 50, 5503–5505 RSC.
- S. J. Lin and P. Theato, Macromol. Rapid Commun., 2013, 34, 1118–1133 CrossRef CAS PubMed.
- Q. Yan, R. Zhou, C. K. Fu, H. J. Zhang, Y. W. Yin and J. Y. Yuan, Angew. Chem., Int. Ed., 2011, 50, 4923–4927 CrossRef CAS PubMed.
- D. Witt, R. Klajn, P. Barski and B. A. Grzybowski, Curr. Org. Chem., 2004, 8, 1763–1797 CrossRef CAS.
- R. Klajn, Pure Appl. Chem., 2010, 82, 2247–2279 CrossRef.
- A. R. Rothrock, R. L. Donkers and M. H. Schoenfisch, J. Am. Chem. Soc., 2005, 127, 9362–9363 CrossRef CAS PubMed.
- S. Zhou, X. Chen, T. Nguyen, A. K. Voice and G. T. Rochelle, ChemSusChem, 2010, 3, 913–918 CrossRef CAS PubMed.
- N. H. Khdary and M. A. Ghanem, J. Mater. Chem., 2012, 22, 12032–12038 RSC.
- S. Kadiwala, A. V. Rayer and A. Henni, Chem. Eng. J., 2012, 179, 262–271 CrossRef CAS PubMed.
- J. Alauzun, A. Mehdi, C. Reye and R. J. P. Corriu, J. Am. Chem. Soc., 2005, 127, 11204–11205 CrossRef CAS PubMed.
- Under our experimental conditions, transformation of the carbamate into a cyclic urea is highly unlikely as it typically requires high CO2 pressures, elevated temperatures, and/or the presence of a catalyst (e.g.ref. 38 and 39).
- M. Tamura, K. Noro, M. Honda, Y. Nakagawa and K. Tomishige, Green Chem., 2013, 15, 1567–1577 RSC.
- C. Wu, H. Cheng, R. Liu, Q. Wang, Y. Hao, Y. Yu and F. Zhao, Green Chem., 2010, 12, 1811–1816 RSC.
- This conclusion is supported by an experiment in which we incubated AuNPs co-functionalized with 3 and C12SH under oxygen for an extended period of time. Under oxidative conditions, the presence of free SH groups on the outer surfaces of NPs would likely cause crosslinking due to disulfide bridge formation. No such crosslinking was observed by DLS or UV-Vis spectroscopy.
- T. Zdobinsky, P. S. Maiti and R. Klajn, J. Am. Chem. Soc., 2014, 136, 2711–2714 CrossRef PubMed.
- In addition to the increasing NP surface polarity in the nonpolar medium, attractive forces between the NPs could originate from direct interactions (e.g. due to dipole–dipole interactions of the cis-azobenzene dipoles).
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis and characterisation of ligand 3; synthesis and functionalisation of gold nanoparticles; and control experiments. See DOI: 10.1039/c4cc08541h |
| This journal is © The Royal Society of Chemistry 2015 |