Sulphur promoted C(sp3)–C(sp2) cross dehydrogenative cyclisation of acetophenone hydrazones with aldehydes: efficient synthesis of 3,4,5-trisubstituted 1H-pyrazoles†
Abstract
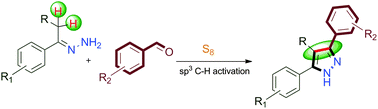
A novel strategy for the cross dehydrogenative coupling (CDC) of acetophenone hydrazones and aldehydes has been developed for the synthesis of highly substituted pyrazoles. This work, for the first time, uses elemental sulfur as a promoter as well as a hydrogen acceptor in effecting the Csp3–Csp2 bond formation via C–H activation.
- This article is part of the themed collection: 20th Anniversary of the CRSI: Celebrating Indian Chemistry in ChemComm

 Please wait while we load your content...
Please wait while we load your content...