Open Access Article
Open Access ArticleSynthesis of polysubstituted cyclopenta[b]indoles via relay gold(I)/Brønsted acid catalysis†
Seema
Dhiman
and
S. S. V.
Ramasastry
*
Department of Chemical Sciences, Indian Institute of Science Education and Research (IISER) Mohali, Sector 81, Manuali PO, S. A. S. Nagar, Punjab 140306, India. E-mail: ramsastry@iisermohali.ac.in
First published on 6th November 2014
Abstract
An efficient relay catalytic process involving Au(I)/Brønsted acid to access various polysubstituted cyclopentannulated indoles from easily accessible 1-(2-aminophenyl)prop-2-ynols and readily available 1,3-dicarbonyls has been developed. In an unprecedented event, the intermediate 2-indolylmethyl cations undergo the cation-Ene reaction with various 1,3-dicarbonyls followed by an intramolecular Friedel–Crafts-type reaction generating functionalized cyclopenta[b]indoles.
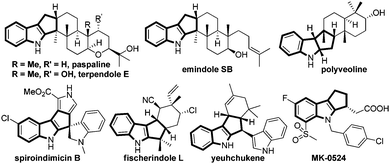
Indoles and indolines are considered to be privileged structures due to their widespread occurrence in Nature with intricate structural diversity often associated with impressive bioactivities, and in a pharmaceutical sense due to their drug-like properties.1 Among indole derivatives, cyclopenta[b]indoles are especially attractive due to their presence in numerous biologically active natural products, for example, paspalines, terpendoles, emindoles, polyveolines, spiroindimicins, fischerindoles, yuehchukene, etc., in addition to medicinally important compounds such as MK-0524, Fig. 1.2 The presence of complex molecular architectures coupled with impressive pharmacological properties prompted several research groups to contribute significantly to the construction of cyclopentannulated indole derivatives.3 However, the quest for the development of a simple and efficient method to access this class of compounds from readily available starting materials still remains an area of active research.
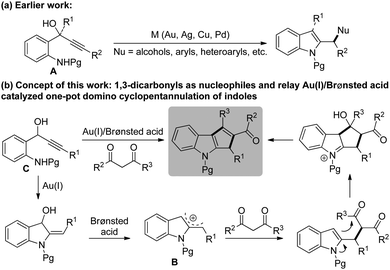
On the other hand, activation of π-systems of alkynes and alkenes via gold catalysis for the synthesis of a wide range of natural products and complex molecules in an efficient and predictable manner has received significant attention during the past decade.4 Especially, relay catalytic processes involving gold were demonstrated to have great potential to rapidly assemble complex chemical structures often associated with pot, step and atom economy.5 Motivated by the pioneering studies by Chan6 for the synthesis of indole derivatives starting from propargylic tert-alcohols of the type A, and with our experience in the chemistry of heteroaryl carbinols,7 we initiated a program to develop an efficient and general methodology towards the synthesis of a novel series of cyclopentannulated indoles and to evaluate their biological efficacy, Scheme 1. Prior to commencing our investigation, a detailed literature survey revealed that the prevailing 2-indolylmethyl cation intermediate B was routinely trapped by nucleophiles such as alcohols, aryls, heteroaryls, etc.8 However, to our surprise, no attempt was ever made to employ readily available 1,3-dicarbonyl compounds as nucleophiles.9
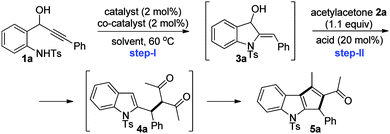
Herein, we delineate our efforts towards the generation of indolylmethyl cations from 2-aminophenyl propargylic secondary alcohols C,10 their reactions with a variety of 1,3-dicarbonyl compounds and subsequent intramolecular aldol-type reactions for the synthesis of 1,2,3-trisubstituted cyclopenta[b]indoles. Accordingly, we initiated optimization studies towards identifying an effective catalytic system and other reaction parameters. For this, amino alcohol 1a6b,11 and acetylacetone 2a were chosen as substrates for the model reaction. The screening results are compiled in Table 1.
| Entry | Catalyst | Co-catalyst | Acid | Solvent | Time (h) | Yieldb (%) |
|---|---|---|---|---|---|---|
| a Reaction conditions: a 5 mL glass vial was filled with 1a (0.1 mmol), catalyst (2 mol%), co-catalyst (2 mol%) in an appropriate solvent (1 mL) and stirred at 60 °C, upon disappearance of the starting compound (1a), acetylacetone 2a (0.11 mmol) and an acid (20 mol%) were introduced and stirring was continued at 60 °C until indoline 3a and acetylacetone adduct 4a disappeared. b Isolated yields after silica gel column chromatography. c Intermediate 3a formed and only the acetylacetone adduct 4a was isolated. d 10 mol% TfOH was employed. e Step-II was carried out at room temperature. | ||||||
| 1 | AuCl | — | — | DCE | 48 | — |
| 2 | PPh3AuCl | — | — | DCE | 48 | — |
| 3 | AuCl | AgOTf | — | DCE | 48 | — |
| 4 | PPh3AuCl | AgOTf | — | DCE | 48 | — |
| 5c | AuCl | K2CO3 | Sc(OTf)3 | MeNO2 | 48 | — |
| 6c | AuCl | K2CO3 | In(OTf)3 | MeNO2 | 48 | — |
| 7c | AuCl | K2CO3 | AgOTf | MeNO2 | 48 | — |
| 8 | AuCl | K2CO3 | Bi(OTf)3 | MeNO2 | 26 | 74 |
| 9 | AuCl | K2CO3 | TMSOTf | DCE | 20 | 82 |
| 10 | AuCl | K2CO3 | TFA | DCE | 13 | — |
| 11 | AuCl | K2CO3 | H3PO4 | DCE | 16 | 51 |
| 12 | AuCl | K2CO3 | HClO4 | DCE | 15 | 72 |
| 13 | AuCl | K2CO3 | H2SO4 | DCE | 18 | 70 |
| 14 | AuCl | K2CO3 | TfOH | DCE | 13 | 83 |
| 15 | AuCl | K2CO3 | TfOH | MeNO2 | 24 | 74 |
| 16 | AuCl | K2CO3 | TfOH | Toluene | 13 | 80 |
| 17 | AuCl | NaHCO3 | — | DCE | 40 | — |
| 18 | AuCl | Na2CO3 | TfOH | DCE | 35 | 53 |
| 19 | AuCl | Et3N | TfOH | DEC | 27 | 72 |
| 20 | AuCl | K2CO3 | TfOH | DCE | 18 | 90 |
| 21c,e | AuCl | K2CO3 | TfOH | DCE | 48 | — |
| 22d | PPh3AuCl | K2CO3 | TfOH | DCE | 42 | 76 |
| 23d | AuCl | K2CO3 | TMSOTf | DCE | 36 | 85 |
| 24d | AgOTf | — | TfOH | MeNO2 | 34 | 63 |
To begin with, cyclization of amino alcohol 1a to indoline 3a was explored. The reaction of 1a in the presence of Au(I) or Ag(I) salts alone or a combination of Au(I) and silver based Lewis acids failed to deliver indoline 3a (Table 1, entries 1–4). Interestingly, amino alcohol 1a generated indoline 3a when a combination of Au(I) and base was employed;12 however, subsequent transformation of indoline 3a to the desired indole 5a was not observed upon treatment with a variety of Lewis acids, only the acetylacetone adduct 4a was isolated (Table 1, entries 5–7). Nevertheless, an approach for indoline 3avia a catalytic sequence involving Au(I) and a base could be established for the first time.13
Upon further screening, Lewis acids such as Bi(OTf)3 and TMSOTf gratifyingly generated the cyclopentannulated indole 5a in very good yields (Table 1, entries 8 and 9). We were delighted especially because, as per our hypothesis, the intervening indolylmethyl cation formed under Lewis acidic conditions successfully underwent the cation-Ene reaction with acetylacetone 2a, and furthermore the 1,3-dicarbonyl adduct 4avia an intramolecular Friedel–Crafts-type reaction furnished 1,2,3-trisubstituted cyclopenta[b]indole 5a. It is worth mentioning that the current method also constitutes a potential alternative to the traditionally employed methods for indole cyclopentannulations such as [3+2]-cycloadditions, Nazarov cyclizations, etc.14 Furthermore, synthesis of cyclopentannulated indoles via 2-indolylmethyl cation intermediates is underexplored.3h
In order to further improve the efficiency of the reaction, we opted to investigate the influence of Brønsted acids in place of Lewis acids in step-II. Among several Brønsted acids explored, trifilic acid mediated reaction in DCE delivered the cyclopentannulated indole 5a in very good yield (Table 1, entries 10–16). Subsequently, influence of the co-catalyst (base) in step-I was also studied. While no desired product was observed when sodium bicarbonate was employed as the co-catalyst, sodium carbonate and an organic base such as triethylamine furnished 5a only in moderate yields (Table 1, entries 17–19). Gratifyingly, further enhancement in the yield was observed when TfOH loading was reduced from 20 mol% to 10 mol% (Table 1, entry 20). Striking temperature dependence was realized when the TfOH reaction at room temperature failed to generate the desired product 5a and only the acetylacetone adduct 4a was isolated (Table 1, entry 21). Further attempts to enhance the yield were not encouraging (Table 1, entries 22–24).
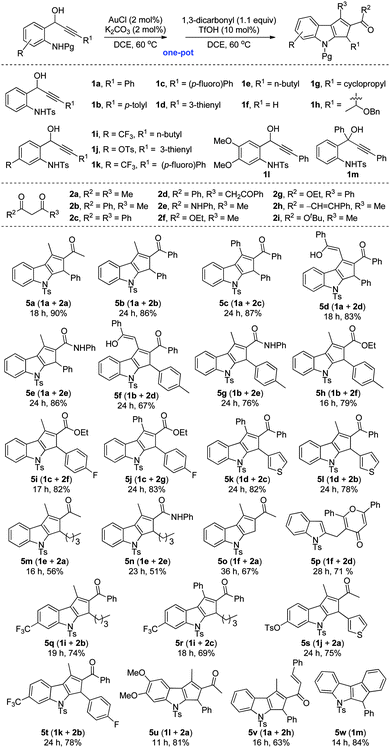
In order to validate the generality of the unprecedented method for the synthesis of polysubstituted cyclopenta[b]indoles, a variety of 1-(2-aminophenyl)prop-2-ynols 1a–1m were synthesized according to literature methods6,8 and subjected to the optimized conditions, Table 2. It is noteworthy that the relay Au(I)/Brønsted acid catalyzed tandem transformation is quite general, and a diverse library of annulated indoles can be rapidly accessed in good to excellent yields (Table 2, 5a–5w). The reaction displays significant tolerance towards various alkynols bearing electron-donating as well as electron-withdrawing aryl groups (for example, p-tolyl and p-fluorophenyl), heteroaryls such as 2-thienyl, and the alkyl groups. Furthermore, alkyl and aryl-bearing 1,3-diketones, 1,3,5-triketones, β-ketoesters and β-ketoamides are also well-tolerated under the reaction conditions.
| a Reaction conditions: a mixture of amino alcohol 1 (0.1 mmol), AuCl (2 mol%), K2CO3 (2 mol%) and DCE (1 mL) in a 5 mL glass vial was stirred at 60 °C. After complete consumption of the starting compound (1), 1,3-dicarbonyl 2 (0.11 mmol) and TfOH (10 mol%) were added successively and stirring was continued at 60 °C until the complete disappearance of the respective 1,3-dicarbonyl adduct (4). b Isolated yields after silica gel column chromatography. |
|---|
|
As can be seen from Table 2, amino alcohol 1a upon reaction with di- and triketones 2a–2d, and ketoamide 2e generated the respective 1,2,3-trisubstituted cyclopentannulated indoles 5a–5e in excellent yields. Similarly, the analogous aryl and heteroaryl amino alcohols 1b–1d upon reaction with a variety of diketones, ketoesters and ketoamides generated functionalized cyclopenta[b]indoles (Table 2, 5f–5l). Significantly, reaction of 1e, having a pendent alkyl group on the acetylenic carbon centre, with diketones 2a and 2e also furnished the respective annulated indoles 5m and 5n though in moderate yields,15 but enhanced the scope of this method. Even 1,2-disubstituted cyclopentannulated indoles (such as 5o) could be efficiently generated by the reaction of unsubstituted alkynol 1f with diketone 2a. But reaction of 1f with triketone 2d, unexpectedly formed the pyranone indole 5p. Complex indole derivatives bearing electron-withdrawing as well as electron-donating substituents (such as –CF3, –OTs, –OMe) on the indole moiety could also be accessed easily in high yields (Table 2, 5q–5u). However, contrary to our expectation, the amino alcohols 1g and 1h failed to deliver the desired products under the reaction conditions. Presumably, the presence of an acid sensitive cyclopropyl system and a 2-butyne-1,4-oxygenated system would have triggered unwanted side reactions. The molecular structure of a representative example 5v, obtained by the reaction of 1a and 2h, was unambiguously confirmed by single crystal X-ray diffraction analysis (see the ESI† for details).16
On the other hand, reaction of amino tert-alcohol 1m even in the presence of 2a generated only indole 5w in 84% yield via a Nazarov electrocyclization reaction (Table 2).6a Thus, indoles of the type 5w could now be accessed in excellent yields with less Au(I) catalyst loading and under milder reaction conditions over the existing method.6a
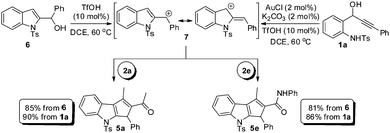
Since alcohol 6 and cationic intermediate 7 are believed to be the intermediates in the transformation of 1a to 5a, we planned to undertake a comparative study between the reactions of amino alcohol 1a and 2-indolyl carbinol 6 under the optimized conditions, Scheme 2. It can be noted that the reaction of amino alcohol 1a with 2a or 2e is found to be efficient in generating 5a or 5e, respectively, when compared to the reaction of alcohol 6 in forming 5a and 5e, thereby clearly demonstrating the advantage of the one-pot tandem process. It is worth mentioning that the direct Friedel–Crafts-type alkylation of unmodified 2-indolyl carbinols and 1,3-dicarbonyls as such is unprecedented17 and of course the subsequent cyclization cascade as well.
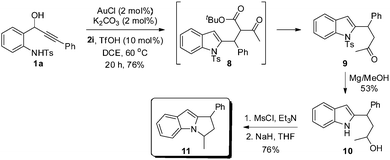
To further illustrate the generality and synthetic utility of this methodology, we considered an elaboration (see Scheme 3). Thus, reaction of 1a with ketoester 2i under the optimized conditions furnished adduct 8, which underwent smooth in situ decarboxylation to form β-branched 4-(2-indolyl)-2-butanone 9 in 76% yield, synthesis of which otherwise would require a multistep sequence. Indole 9 upon reaction with excess Mg in methanol generated alcohol 10 by undergoing simultaneous tosyl deprotection and ketone reduction. Selective O-mesylation and subsequent intramolecular N-alkylation18 conveniently generated 1,3-disubstituted dihydropyrroloindole 11, an important motif prevalent in a number of pharmaceutically important compounds and natural products.19
In conclusion, we have developed a general and efficient relay Au(I)/Brønsted acid catalyzed one-pot tandem process for the synthesis of medicinally significant 1,2-di- and 1,2,3-trisubstituted cyclopentannulated indoles from 1-(2-aminophenyl)prop-2-ynols and 1,3-dicarbonyls. Key features of this method are its readily accessible starting compounds and atom, step and pot economy. During the course of our investigation, we also developed novel Au(I)/base mediated conditions for the synthesis of indolines starting from 1-(2-aminophenyl)prop-2-ynols. In addition, we have demonstrated for the first time, Friedel–Crafts-type alkylation of unmodified 2-indolyl carbinols and 1,3-dicarbonyls. A study regarding the application of this methodology for the synthesis of biologically active natural products is currently underway in our laboratory and will be communicated shortly.
We are grateful to the DST, Govt of India for financial support through the Fast Track Scheme (SR/FT/CS-156/2011). We thank IISER Mohali for funding and for NMR, mass, and X-ray facilities. S.D. thanks IISER Mohali for a research fellowship.
Notes and references
- Some selected reviews: (a) M. Somei and F. Yamada, Nat. Prod. Rep., 2005, 22, 73 RSC; (b) T. Kawasaki and K. Higuchi, Nat. Prod. Rep., 2005, 22, 761 RSC; (c) S. Cacchi and G. Fabrizi, Chem. Rev., 2005, 105, 2873 CrossRef CAS PubMed; (d) K. Higuchi and T. Kawasaki, Nat. Prod. Rep., 2007, 24, 843 RSC; (e) V. Sharma, P. Kumar and D. Pathak, J. Heterocycl. Chem., 2010, 47, 491 CAS.
- For paspalines, terpendoles, lolicines, and lolitrems, see: (a) S. C. Munday-Finch, A. L. Wilkins and C. O. Miles, J. Agric. Food Chem., 1998, 46, 590 CrossRef CAS PubMed ; for emindoles, see:; (b) H. Harms, V. Rempel, S. Kehraus, M. Kaiser, P. Hufendiek, C. E. Muller and G. M. Konig, J. Nat. Prod., 2014, 77, 673 CrossRef CAS PubMed ; for polyveolines, see: ; (c) I. Ngantchou, B. Nyasse, C. Denier, C. Blonski, V. Hannaert and B. Schneider, Bioorg. Med. Chem. Lett., 2010, 20, 3495 CrossRef CAS PubMed ; for spiroindimicins, see:; (d) W. Zhang, Z. Liu, S. Li, T. Yang, Q. Zhang, L. Ma, X. Tian, H. Zhang, C. Huang, S. Zhang, J. Ju, Y. Shen and C. Zhang, Org. Lett., 2012, 14, 3364 CrossRef CAS PubMed ; for fischerindoles, see:; (e) J. M. Richter, Y. Ishihara, T. Masuda, B. W. Whitefield, T. Llamas, A. Pohjakallio and P. S. Baran, J. Am. Chem. Soc., 2008, 130, 17938 CrossRef CAS PubMed ; for yeuhchukene, see: ; (f) Y.-C. Kong, K.-F. Cheng, R. C. Cambie and P. G. Waterman, J. Chem. Soc., Chem. Commun., 1986, 47 Search PubMed.
- Some selected references for the synthesis of the cyclopenta[b]indole frameworks, see: (a) C. A. Harrison, R. Leineweber, C. J. Moody and J. M. J. Williams, J. Chem. Soc., Perkin Trans. 1, 1995, 1127 RSC; (b) M. Ishikura, Y. Matsuzaki and I. Agata, Chem. Commun., 1996, 2409 RSC; (c) E. M. Ferreira and B. M. Stoltz, J. Am. Chem. Soc., 2003, 125, 9578 CrossRef CAS PubMed; (d) C. Venkatesh, P. P. Singh, H. Ila and H. Junjappa, Eur. J. Org. Chem., 2006, 5378 CrossRef CAS; (e) K. S. Feldman, M. R. Iyer and D. K. Hester II, Org. Lett., 2006, 8, 3113 CrossRef CAS PubMed; (f) M. G. Banwell, X. Ma, R. M. Taylor and A. C. Willis, Org. Lett., 2006, 8, 4959 CrossRef CAS PubMed; (g) J. A. Malona, J. M. Colbourne and A. J. Frontier, Org. Lett., 2006, 8, 5661 CrossRef CAS PubMed; (h) A. K. Yadav, S. Peruncheralathan, H. Ila and H. Junjappa, J. Org. Chem., 2007, 72, 1388 CrossRef CAS PubMed; (i) C. Ferrer, C. H. M. Amijs and A. M. Echavarren, Chem. – Eur. J., 2007, 13, 1358 CrossRef CAS PubMed; (j) E. P. Balskus and C. T. Walsh, J. Am. Chem. Soc., 2009, 131, 14648 CrossRef CAS PubMed; (k) N.-W. Tseng and M. Lautens, J. Org. Chem., 2009, 74, 1809 CrossRef CAS PubMed; (l) F. Churruca, M. Fousteris, Y. Ishikawa, M. von Wantoch Rekowski, C. Hounsou, T. Surrey and A. Giannis, Org. Lett., 2010, 12, 2096 CrossRef CAS PubMed; (m) K. Saito, H. Sogou, T. Suga, H. Kusama and N. Iwasawa, J. Am. Chem. Soc., 2011, 133, 689 CrossRef CAS PubMed; (n) B. Chen, W. Fan, G. Chai and S. Ma, Org. Lett., 2012, 14, 3616 CrossRef CAS PubMed; (o) B. Xu, Z.-L. Guo, W.-Y. Jin, Z.-P. Wang, Y.-G. Peng and Q.-X. Guo, Angew. Chem., Int. Ed., 2012, 51, 1059 CrossRef CAS PubMed.
- For selected articles on relay gold catalysis, see: (a) G. Dyker, Angew. Chem., Int. Ed., 2000, 39, 4237 CrossRef CAS; (b) G. C. Bond, Catal. Today, 2002, 72, 5 CrossRef CAS; (c) M. Haruta, Nature, 2005, 437, 1098 CrossRef CAS PubMed; (d) A. S. K. Hashmi and G. J. Hutchings, Angew. Chem., Int. Ed., 2006, 45, 7896 CrossRef PubMed; (e) Z. Li, C. Brouwer and C. He, Chem. Rev., 2008, 108, 3239 CrossRef CAS PubMed; (f) A. Arcadi, Chem. Rev., 2008, 108, 3266 CrossRef CAS PubMed; (g) D. J. Gorin, B. D. Sherry and F. D. Toste, Chem. Rev., 2008, 108, 3351 CrossRef CAS PubMed; (h) A. S. K. Hashmi and M. Rudolph, Chem. Soc. Rev., 2008, 37, 1766 RSC; (i) A. Furstner, Chem. Soc. Rev., 2009, 38, 3208 RSC; (j) M. Bandini, Chem. Soc. Rev., 2011, 40, 1358 RSC; (k) D. Qian and J. Zhang, Chem. – Eur. J., 2013, 19, 6984 CrossRef CAS PubMed; (l) H. Wu, Y.-P. He and L.-Z. Gong, Org. Lett., 2013, 15, 460 CrossRef CAS PubMed; (m) P.-S. Wang, K.-N. Li, X.-L. Zhou, X. Wu, Z.-Y. Han, R. Guo and L.-Z. Gong, Chem. – Eur. J., 2013, 19, 6234 CrossRef CAS PubMed; (n) X. Wu, M.-L. Li and P.-S. Wang, J. Org. Chem., 2014, 79, 419 CrossRef CAS PubMed; (o) C. Obradorsa and A. M. Echavarren, Chem. Commun., 2014, 50, 16 RSC; (p) Y. Horino, Y. Takahashi, Y. Nakashima and H. Abe, RSC Adv., 2014, 4, 6215 RSC; (q) S. Nayak, N. Ghosh and A. K. Sahoo, Org. Lett., 2014, 16, 2996 CrossRef CAS PubMed.
- (a) N. T. Patil, V. S. Shinde and B. Gajula, Org. Biomol. Chem., 2012, 10, 211 RSC; (b) M. Rueping, J. Dufour and M. S. Maji, Chem. Commun., 2012, 48, 3406 RSC.
- (a) P. Kothandaraman, W. Rao, S. J. Foo and P. W. H. Chan, Angew. Chem., Int. Ed., 2010, 49, 4619 CrossRef CAS PubMed; (b) D. Susanti, F. Koh, J. A. Kusuma, P. Kothandaraman and P. W. H. Chan, J. Org. Chem., 2012, 77, 7166 CrossRef CAS PubMed; (c) S. R. Mothe, P. Kothandaraman, S. J. L. Lauw, S. M. W. Chin and P. W. H. Chan, Chem. – Eur. J., 2012, 18, 6133 CrossRef CAS PubMed.
- (a) S. Dhiman and S. S. V. Ramasastry, Org. Biomol. Chem., 2013, 11, 4299 RSC; (b) S. Dhiman and S. S. V. Ramasastry, Org. Biomol. Chem., 2013, 11, 8030 RSC; (c) S. Dhiman and S. S. V. Ramasastry, J. Org. Chem., 2013, 78, 10427 CrossRef CAS PubMed; (d) B. Satpathi, S. Dhiman and S. S. V. Ramasastry, Eur. J. Org. Chem., 2014, 2022 CrossRef CAS; (e) S. Kasare, S. K. Bankar and S. S. V. Ramasastry, Org. Lett., 2014, 16, 4284 CrossRef CAS PubMed.
- (a) C. Chowdhury, B. Das, S. Mukherjee and B. Achari, J. Org. Chem., 2012, 77, 5108 CrossRef CAS PubMed; (b) N. Thirupathi, M. H. Babu, V. Dwivedi, R. Kant and M. S. Reddy, Org. Lett., 2014, 16, 2908 CrossRef CAS PubMed; (c) H. Li, X. Li, H.-Y. Wang, G. N. Winston-McPherson, H.-M. J. Geng, I. A. Guzei and W. Tang, Chem. Commun., 2014, 50, 12293 RSC.
- As such, reactions of 1,3-dicarbonyls and 2-indolylmethyl cations generated by any method were never studied.
- While reactions of 2-indolylmethyl cations originating from propargylic tertiary alcohols (A) with different nucleophiles are well-established,6,8 generation of 2-indolylmethyl cations from propargylic secondary alcohols (C) and their reactions with any nucleophile have not been reported so far.
- Other NH-protecting groups (Ms, Boc, and Ac) were also evaluated prior to proceeding to optimization. Only N-sulfonyl propargyl alcohols generated the desired product (see the ESI† for details).
- Step-I was found to proceed very slowly at room temperature. After several attempts, the reaction temperature was optimized to 60 °C.
- Earlier, Au(I)/Lewis acid,6a Ag(I),6b Pd,8a,b Cu,8c conditions were reported for the conversion of 1 to 3. For Au(I)/base mediated cyclization of 2-(1-hydroxyprop-2-ynyl)phenols to dihydrobenzo-furans, see: H. Harkat, A. Blanc, J.-M. Weibel and P. Pale, J. Org. Chem., 2008, 73, 1620 CrossRef CAS PubMed.
- For [3+2]-cycloadditions, see: (a) I. Kawasaki, M. Terano, A. Kurume, S. Hara, M. Yamashita and S. Ohta, Tetrahedron Lett., 2005, 46, 6549 CrossRef CAS; (b) J. McNulty and D. McLeod, Synlett, 2011, 717 CrossRef CAS; (c) H. Li, R. P. Hughes and J. Wu, J. Am. Chem. Soc., 2014, 136, 6288 CrossRef CAS PubMed . Also see: ref. 3a, d, k and m. For Nazarov cyclizations, see: ; (d) J. A. Jordan, G. W. Gribble and J. C. Badenock, Tetrahedron Lett., 2011, 52, 6772 CrossRef CAS . Also see: ref. 3g and l.
- Yield loss in general is attributed to the formation of 2-vinyl-1H-indoles in case of substrates with a pendent alkyl group on the acetylenic carbon centre.6a.
- CCDC 1029309 has been assigned.
- For indirect, SN2-type reactions, see: (a) M. C. Hillier, J. F. Marcoux, D. L. Zhao, E. J. Grabowski, A. E. McKeown and R. D. Tillyer, J. Org. Chem., 2005, 70, 8385 CrossRef CAS PubMed; (b) M. Bandini and A. Eichholzer, Angew. Chem., Int. Ed., 2009, 48, 9533 CrossRef CAS PubMed.
- A. Kuznetsov, A. Makarov, A. E. Rubtsov, A. V. Butin and V. Gevorgyan, J. Org. Chem., 2013, 78, 12144 CrossRef CAS PubMed.
- For pharmaceutically relevant compounds, see: (a) Z. Ding and N. Yoshikai, Angew. Chem., Int. Ed., 2013, 52, 8574 CrossRef CAS PubMed ; for bioactive natural products, see: ; (b) D. H. Dethe, R. D. Erande and B. D. Dherange, Org. Lett., 2014, 16, 2764 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Complete experimental procedures and characterization data of all new compounds. CCDC 1029309. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4cc08174a |
| This journal is © The Royal Society of Chemistry 2015 |