Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A simple modular aptasensor platform utilizing cucurbit[7]uril and a ferrocene derivative as an ultrastable supramolecular linker†
Don-Wook
Lee‡
ab,
Kyeng Min
Park‡
a,
Bokyoung
Gong
ac,
Dinesh
Shetty
a,
Jayshree K.
Khedkar
a,
Kangkyun
Baek
a,
Jeehong
Kim
ac,
Sung Ho
Ryu
*bd and
Kimoon
Kim
*abce
aCenter for Self-assembly and Complexity, Institute for Basic Science (IBS), Pohang, 790-784, Republic of Korea
bSchool of Interdisciplinary Bioscience and Bioengineering, Pohang University of Science and Technology, Pohang, 790-784, Republic of Korea. E-mail: kkim@postech.ac.kr
cDepartment of Chemistry, Pohang University of Science and Technology, Pohang, 790-784, Republic of Korea
dDivision of molecular and Life Science, Department of Life Science, Pohang University of Science and Technology, Pohang, 790-784, Republic of Korea
eDivision of Advanced Materials Science, Pohang University of Science and Technology, Pohang, 790-784, Republic of Korea
First published on 5th January 2015
Abstract
A simple modular aptamer-based sensor (aptasensor) platform was prepared by combining the merits of the rapid and efficient preparation of a self-assembled monolayer of cucurbit[7]uril (CB[7] SAM) and the strong and specific binding affinity of CB[7] to ferrocenemethylammonium (FA), as an ultrastable supramolecular linker, to immobilize aptamers on CB[7] SAM.
Aptamers are the artificial nucleotide sequences that fold into secondary and tertiary structures with high selectivity and specificity to target molecules including proteins, peptides and small organic molecules.1 Along with these features, aptamers have shown extraordinary promise in analytical applications due to their easy chemical synthesis and modifications with reporter molecules, linkers, and other functional groups.2 Many researchers have thus explored the aptamers as highly selective biorecognition components in bioassays and biosensing.2,3 Apart from the inherent advantages of biosensors, aptamer-based biosensors (aptasensors) offer the advantage of reusability utilizing reversible structural-switching.4 Furthermore, their small size and chemical functionalities allow efficient immobilization at high density,5 which is of vital importance in multiplexing miniaturized systems. In spite of these rapid advances, aptamer-based bioassays are still immature compared to antibody-based immunoassays, thus reflecting the limited availability of aptamer types and the relatively poor knowledge of surface-immobilization technologies for aptamers.6 In this regard, the multiple processing steps for immobilization of aptamers on surfaces is a major concern in the development of such systems.7 The streptavidin–biotin binding pair8 has extensively been used as an efficient linker system to immobilize aptamers on surfaces5b,9 because of their strong and specific noncovalent interactions (binding constant ∼1013 M−1). The multiple processing steps include the pre-treatment of additional chemical reagents, such as alkane thiols, functionalized dextrans, polyethyleneglycol thiols (PEG-SH) and also requires a chemical modification of proteins with delicate handling.7,10 Each step in this process claims to be a crucial parameter in the analytical performance of aptasensors. Such limitations therefore necessitate a new aptasensor platform that can simplify the processing steps using a chemically stable binding pair which could be convenient in terms of storage and handling.
Earlier, we and other researchers reported that cucurbit[7]uril (CB[7]), a member of the host family cucurbit[n]uril (CB[n], n = 5–8, 10, 14)11,12 with a hydrophobic cavity and two identical carbonyl-fringed portals, binds the ferrocenemethylammonium (FA) ion with an exceptionally high binding affinity with a binding constant of ∼1012 M−1 in aqueous solution.13 This exceptionally strong binding affinity of CB[7] to FA led us to develop a synthetic system successfully employed, as an alternative artificial system to a streptavidin–biotin binding pair, in biological applications such as immobilization of biomolecules on a solid surface and membrane protein isolation.14 Recently, Li and others reported a method for the formation of a self-assembled monolayer of CB[7] on a gold surface by dipping a gold surface into a solution of CB[7].15 It was observed that CB[7] molecules on the gold surface are uniform in their orientation and hold carbonyl portals perpendicular to the plane of the surface to maintain the guest binding and recognition properties of CB[7]. Further, Brunsveld et al. demonstrated simple and reversible immobilization of FA-conjugated yellow fluorescent protein (FA-YFP) on CB[7].16 Although aptamers have become increasingly important molecular tools in the biosensing field because of the inherent advantages mentioned above, the immobilization of aptamers on CB[7] has not been reported yet.
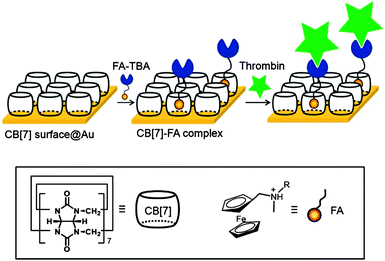
Described herein is a simple modular aptasensor platform obtained by combining the merits of both simple preparation of CB[7] SAM and highly strong and selective binding affinity of CB[7] to FA. We utilize the FA–CB[7] binding pair system as an ultrastable supramolecular linker, with the help of which various FA-conjugated aptamers can efficiently be immobilized on CB[7] SAM for surface plasmon resonance (SPR) studies (Scheme 1). Our present aptasensor system bears unique features including (1) easy preparation of CB[7] SAM by either direct injection of CB[7] onto a gold surface or dipping a gold surface into a solution of CB[7], (2) versatility of CB[7] SAM for immobilization of various FA-conjugated aptamers, (3) resistance to harsh conditions such as elevated temperature or unwanted enzymatic degradation, and (4) long-term durability due to the usage of stable synthetic molecules,14b instead of (strept)avidin proteins used in conventional aptasensors. This FA–CB[7] linker-based aptasensor may act as a simple and efficient bio-sensor including proteins and thus widen the practical applications of aptasensors.
CB[7] SAM was prepared by simply dipping a gold surface into a solution of CB[7] (1.0 mM in deionized H2O) for 6 h as per the literature reports15a and conveniently kept at RT until it was ready to be used as a sensor chip. Direct injections of CB[7] solution (1.0 mM in deionized H2O, 20 min, 20 µL min−1) onto a gold surface mounted in a SPR machine were also conducted as an alternative method. After 5 sequential injections, we observed approximately 500 RU (1000 RU = 1 ng mm−2)17 of sensor response (Fig. S1, ESI†) which turned out to be 0.43 pmol of CB[7] on 1 mm2. This result suggested that ca. 55% of a plain gold surface is covered with CB[7] by considering the theoretical density of densely packed CB[7] (of diameter 1.6 nm)12b on a plane surface (0.75 pmol mm−2). In AFM images (Fig. S2, ESI†), small dots approximately 1 nm in height and only little aggregation appeared on a gold surface after treatment of CB[7]. The SPR and AFM results suggest that even simple direct injections of CB[7] solution to a gold surface can lead to the formation of CB[7] SAM on a gold sensor chip. Since CB[7] is a stable molecule synthesized under highly acidic conditions at high temperature,11a,b and it has higher resistance to harsh conditions and better long-term durability compared to (strept)avidin, which suggests that CB[7] SAM can be useful as a robust aptasensor platform.
In the present work, a carefully studied aptamer (TBA, 15-mer DNA-based) that binds strongly and selectively to thrombin was chosen as the model probe.18 By exploiting FA–CB[7] interactions for the immobilization of TBA on CB[7] SAM, it was first “ferrocenylated”14a by conjugation of carboxylated FA (1)14b to aminohexyl group at the 5′ terminus using EDC (1-ethyl-3-(3-dimethylaminopropyl)carbodiimide) coupling (Scheme 2). Thus formed ferrocenylated TBA (FA-TBA) was further purified and characterized using HPLC and ESI-mass spectroscopy, respectively (Fig. S4, ESI†). A binding constant of chemically modified TBA (∼109 M−1) measured via a filter binding assay19 was noted to be comparable to that of unmodified TBA. This implies that the ferrocenylation barely affects the binding affinity of TBA to its target protein, thrombin.20
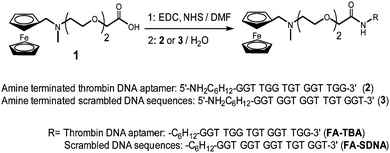 | ||
| Scheme 2 Conjugation of ferrocenemethylammonium to thrombin binding aptamer (FA-TBA) and scrambled DNA sequences (FA-SDNA). | ||
After 25 min of injection of FA-TBA and TBA into each channel on the CB[7] sensor chip, ∼700 RU and 30 RU, respectively, were observed at 1920 s (Fig. 1). The higher RU value (∼23-fold) for FA-TBA compared to TBA proves its importance for effective immobilization of aptamers. The converted density of FA-TBA on CB[7] SAM from the RU value (0.12 pmol mm−2), suggested that approximately one out of four CB[7] molecules on average seemed to capture one FA-TBA. This result suggested successful immobilization of FA-TBA on CB[7] SAM by making the use of strong and specific interactions between CB[7] and FA. To check the versatility of CB[7] SAM for immobilization of various aptamers, we prepared FA-scrambled DNA (FA-SDNA) with the same molecular weight as FA-TBA but with different DNA sequences (Scheme 2). The observed RU value i.e. 670, for FA-SDNA injection was almost in line with that of FA-TBA. This finding clearly shows that CB[7] SAM can not only immobilize FA-TBA but also other various aptamers as long as they are conjugated with FA utilizing the strong and specific interaction between CB[7] and FA as a supramolecular linker.
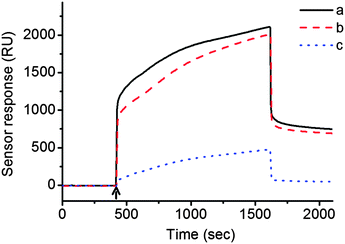 | ||
| Fig. 1 SPR sensorgrams of CB[7] SAM treated with (a) FA-TBA, (b) FA-SDNA and (c) TBA (the arrow indicates injection of samples). | ||
Having established FA-aptamer@CB[7] SAM as a simple and modular aptasensor system, we examined the feasibility of FA-TBA@CB[7] SAM as a model SPR sensor chip. To confirm the selective recognition of thrombin, we injected it and bovine serum albumin (BSA, as a negative control) into a different channel on the FA-TBA@CB[7] sensor chip. Accordingly, after 15 min of injection, we observed 340 RU for thrombin and 35 RU for BSA (Fig. S5, ESI†). These values signify the specific recognition and non-specific adsorption, respectively. Moreover, the higher sensor response of thrombin as compared to BSA, by one order of magnitude, denotes its selective recognition. Furthermore, a concentration dependent sensor response was observed by injection of different thrombin concentrations to a channel of the FA-TBA@CB[7] sensor chip (Fig. 2). These recorded responses ranged from the sub-ppm to the ppm level, thus showing the sensing of target protein in nanomolar concentrations. Even though this noted analytical performance of FA-TBA@CB[7] sensor chip is comparable to that of the streptavidin–biotin binding pair based system reported earlier,7 the former comes up with the benefits of effortless preparation, resistance to harsh conditions, long term durability and reusability by utilizing either reversible structural-switching of aptamers4 or reversible noncovalent binding properties of CB[7] to FA12g,21 as previously demonstrated for proteins.16
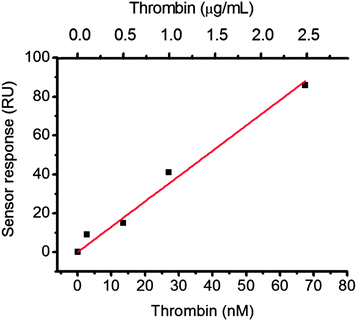 | ||
| Fig. 2 Sensor responses as a function of the thrombin concentration on the CB[7] SAM SPR sensor chip (15 min, 5 µL min−1). | ||
In summary, we have successfully demonstrated the efficient and versatile immobilization of FA-conjugated aptamers with FA-TBA and FA-SDNA on CB[7] SAM by taking advantage of its facile formation on a gold surface and also ultrastable binding of CB[7] to FA. The profound analysis of a target protein using FA-TBA@CB[7] SAM proved the utility of the FA–CB[7] binding pair as an efficient linker system providing a new aptasensor platform. Furthermore, the optimization of the formation of CB[7] SAM and immobilization of FA-aptamers using functionalized CB[7],22 which can further control the density of CB[7] on a gold surface, may result in the enhanced analytical performance of CB[7]-based aptasensors.
This work was supported by the Institute for Basic Science (IBS) [IBS-R007-D1-2014-a00] and the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (No. 2013R1A2A1A03010110).
Notes and references
- (a) A. B. Iliuk, L. Hu and W. A. Tao, Anal. Chem., 2011, 83, 4440–4452 CrossRef CAS PubMed; (b) D. S. Wilson and J. W. Szostak, Annu. Rev. Biochem., 1999, 68, 611–647 CrossRef CAS PubMed; (c) S. M. Shamah, J. M. Healy and S. T. Cload, Acc. Chem. Res., 2008, 41, 130–138 CrossRef CAS PubMed.
- E. Luzi, M. Minunni, S. Tombelli and M. Mascini, TrAC, Trends Anal. Chem., 2003, 22, 810–818 CrossRef CAS.
- (a) S. D. Jayasena, Clin. Chem., 1999, 45, 1628–1650 CAS; (b) W. Zhou, P. J. Huang, J. Ding and J. Liu, Analyst, 2014, 139, 2627–2640 RSC; (c) S. P. Song, L. H. Wang, J. Li, J. L. Zhao and C. H. Fan, TrAC, Trends Anal. Chem., 2008, 27, 108–117 CrossRef CAS PubMed; (d) W. Mok and Y. F. Li, Sensors, 2008, 8, 7050–7084 CrossRef CAS PubMed; (e) C. Tuerk and L. Gold, Science, 1990, 249, 505–510 CAS; (f) S. Tombelli, M. Minunni and M. Mascini, Biomol. Eng., 2007, 24, 191–200 CrossRef CAS PubMed; (g) K. Sefah, J. A. Phillips, X. Xiong, L. Meng, D. Van Simaeys, H. Chen, J. Martin and W. Tan, Analyst, 2009, 134, 1765–1775 RSC; (h) S. Tombelli, M. Minunni and M. Mascini, Biosens. Bioelectron., 2005, 20, 2424–2434 CrossRef CAS PubMed.
- (a) Y. L. Zhang, Y. Huang, J. H. Jiang, G. L. Shen and R. Q. Yu, J. Am. Chem. Soc., 2007, 129, 15448–15449 CrossRef CAS PubMed; (b) Z. S. Wu, M. M. Guo, S. B. Zhang, C. R. Chen, J. H. Jiang, G. L. Shen and R. Q. Yu, Anal. Chem., 2007, 79, 2933–2939 CrossRef CAS PubMed.
- (a) J. F. Lee, G. M. Stovall and A. D. Ellington, Curr. Opin. Chem. Biol., 2006, 10, 282–289 CrossRef CAS PubMed; (b) M. Liss, B. Petersen, H. Wolf and E. Prohaska, Anal. Chem., 2002, 74, 4488–4495 CrossRef CAS.
- A. Bini, M. Minunni, S. Tombelli, S. Centi and M. Mascini, Anal. Chem., 2007, 79, 3016–3019 CrossRef CAS PubMed.
- V. Ostatna, H. Vaisocherova, J. Homola and T. Hianik, Anal. Bioanal. Chem., 2008, 391, 1861–1869 CrossRef CAS PubMed.
- (a) P. C. Weber, D. H. Ohlendorf, J. J. Wendoloski and F. R. Salemme, Science, 1989, 243, 85–88 CAS; (b) N. M. Green, Methods Enzymol., 1990, 184, 51–67 CAS; (c) C. E. Chivers, A. L. Koner, E. D. Lowe and M. Howarth, Biochem. J., 2011, 435, 55–63 CrossRef CAS PubMed.
- (a) A. Tahiri-Alaoui, L. Frigotto, N. Manville, J. Ibrahim, P. Romby and W. James, Nucleic Acids Res., 2002, 30, e45 CrossRef PubMed; (b) R. Kirby, E. J. Cho, B. Gehrke, T. Bayer, Y. S. Park, D. P. Neikirk, J. T. McDevitt and A. D. Ellington, Anal. Chem., 2004, 76, 4066–4075 CrossRef CAS PubMed.
- (a) D. T. Tran, K. Knez, K. P. Janssen, J. Pollet, D. Spasic and J. Lammertyn, Biosens. Bioelectron., 2013, 43, 245–251 CrossRef CAS PubMed; (b) J. Ashley and S. F. Y. Li, Biosens. Bioelectron., 2013, 48, 126–131 CrossRef CAS PubMed; (c) J. W. Lee, S. J. Sim, S. M. Cho and J. Lee, Biosens. Bioelectron., 2005, 20, 1422–1427 CrossRef CAS PubMed.
- (a) J. Kim, I. S. Jung, S. Y. Kim, E. Lee, J. K. Kang, S. Sakamoto, K. Yamaguchi and K. Kim, J. Am. Chem. Soc., 2000, 122, 540–541 CrossRef CAS; (b) A. Day, A. P. Arnold, R. J. Blanch and B. Snushall, J. Org. Chem., 2001, 66, 8094–8100 CrossRef CAS PubMed; (c) S. Liu, P. Y. Zavalij and L. Isaacs, J. Am. Chem. Soc., 2005, 127, 16798–16799 CrossRef CAS PubMed; (d) X. J. Cheng, L. L. Liang, K. Chen, N. N. Ji, X. Xiao, J. X. Zhang, Y. Q. Zhang, S. F. Xue, Q. J. Zhu, X. L. Ni and Z. Tao, Angew. Chem., Int. Ed., 2013, 52, 7252–7255 CrossRef CAS PubMed.
- (a) K. Kim, Chem. Soc. Rev., 2002, 31, 96–107 RSC; (b) J. W. Lee, S. Samal, N. Selvapalam, H. J. Kim and K. Kim, Acc. Chem. Res., 2003, 36, 621–630 CrossRef CAS PubMed; (c) E. Masson, X. X. Ling, R. Joseph, L. Kyeremeh-Mensah and X. Y. Lu, RSC Adv., 2012, 2, 1213–1247 RSC; (d) S. Gadde and A. E. Kaifer, Curr. Org. Chem., 2011, 15, 27–38 CrossRef CAS; (e) L. Isaacs, Chem. Commun., 2009, 619–629 RSC; (f) J. Lagona, P. Mukhopadhyay, S. Chakrabarti and L. Isaacs, Angew. Chem., Int. Ed., 2005, 44, 4844–4870 CrossRef CAS PubMed; (g) A. E. Kaifer, Acc. Chem. Res., 2014, 47, 2160–2167 CrossRef CAS PubMed.
- (a) W. S. Jeon, K. Moon, S. H. Park, H. Chun, Y. H. Ko, J. Y. Lee, E. S. Lee, S. Samal, N. Selvapalam, M. V. Rekharsky, V. Sindelar, D. Sobransingh, Y. Inoue, A. E. Kaifer and K. Kim, J. Am. Chem. Soc., 2005, 127, 12984–12989 CrossRef CAS PubMed; (b) M. V. Rekharsky, T. Mori, C. Yang, Y. H. Ko, N. Selvapalam, H. Kim, D. Sobransingh, A. E. Kaifer, S. Liu, L. Isaacs, W. Chen, S. Moghaddam, M. K. Gilson, K. Kim and Y. Inoue, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 20737–20742 CrossRef CAS PubMed; (c) S. Liu, C. Ruspic, P. Mukhopadhyay, S. Chakrabarti, P. Y. Zavalij and L. Isaacs, J. Am. Chem. Soc., 2005, 127, 15959–15967 CrossRef CAS PubMed.
- (a) I. Hwang, K. Baek, M. Jung, Y. Kim, K. M. Park, D. W. Lee, N. Selvapalam and K. Kim, J. Am. Chem. Soc., 2007, 129, 4170–4171 CrossRef CAS PubMed; (b) D. W. Lee, K. M. Park, M. Banerjee, S. H. Ha, T. Lee, K. Suh, S. Paul, H. Jung, J. Kim, N. Selvapalam, S. H. Ryu and K. Kim, Nat. Chem., 2011, 3, 154–159 CrossRef CAS PubMed.
- (a) Q. An, G. T. Li, C. G. Tao, Y. Li, Y. G. Wu and W. X. Zhang, Chem. Commun., 2008, 1989–1991 RSC; (b) E. Blanco, C. Quintana, L. Hernandez and P. Hernandez, Electroanalysis, 2013, 25, 263–268 CrossRef CAS; (c) A. Gomez-Casado, P. Jonkheijm and J. Huskens, Langmuir, 2011, 27, 11508–11513 CrossRef CAS PubMed.
- J. F. Young, H. D. Nguyen, L. Yang, J. Huskens, P. Jonkheijm and L. Brunsveld, ChemBioChem, 2010, 11, 180–183 CrossRef CAS PubMed.
- X. Q. Cui, R. J. Pei, X. Z. Wang, F. Yang, Y. Ma, S. J. Dong and X. R. Yang, Biosens. Bioelectron., 2003, 18, 59–67 CrossRef CAS.
- (a) L. C. Bock, L. C. Griffin, J. A. Latham, E. H. Vermaas and J. J. Toole, Nature, 1992, 355, 564–566 CrossRef CAS PubMed; (b) R. F. Macaya, P. Schultze, F. W. Smith, J. A. Roe and J. Feigon, Proc. Natl. Acad. Sci. U. S. A., 1993, 90, 3745–3749 CrossRef CAS.
- R. Conrad and A. D. Ellington, Anal. Biochem., 1996, 242, 261–265 CrossRef CAS PubMed.
- E. Baldrich, A. Restrepo and C. K. O'Sullivan, Anal. Chem., 2004, 76, 7053–7063 CrossRef CAS PubMed.
- (a) D. Sobransingh and A. E. Kaifer, Org. Lett., 2006, 8, 3247–3250 CrossRef CAS PubMed; (b) W. Li and A. E. Kaifer, Langmuir, 2012, 28, 15075–15079 CrossRef CAS PubMed.
- (a) S. Y. Jon, N. Selvapalam, D. H. Oh, J. K. Kang, S. Y. Kim, Y. J. Jeon, J. W. Lee and K. Kim, J. Am. Chem. Soc., 2003, 125, 10186–10187 CrossRef CAS PubMed; (b) K. Kim, N. Selvapalam, Y. H. Ko, K. M. Park, D. Kim and J. Kim, Chem. Soc. Rev., 2007, 36, 267–279 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details of chemical synthesis, SPR measurements and filter binding assays. See DOI: 10.1039/c4cc08027k |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2015 |