An unexpected reaction of 2-alkynylaryldiazonium tetrafluoroborate with sulfur dioxide†
Yong
Luo‡
a,
Xiaolin
Pan‡
a,
Chen
Chen
b,
Liangqing
Yao
*b and
Jie
Wu
*ac
aDepartment of Chemistry, Fudan University, 220 Handan Road, Shanghai 200433, China. E-mail: jie_wu@fudan.edu.cn; Fax: +86 21 6564 1740; Tel: +86 21 6510 2412
bObstetrics and Gynecology Hospital, Fudan University, 419 Fangxie Road, Shanghai 200011, China. E-mail: yaoliangqingcn@126.com
cState Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 354 Fenglin Road, Shanghai 200032, China
First published on 30th October 2014
Abstract
An unexpected result from the reaction of 2-alkynylaryldiazonium tetrafluoroborate with sulfur dioxide is described. In the presence of morpholin-4-amine, this transformation catalyzed by copper(I) bromide proceeds through insertion of sulfur dioxide and intramolecular 5-endo cyclization, leading to benzo[b]thiophene 1,1-dioxides in moderate to good yields.
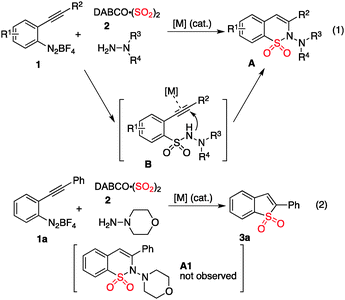
Currently, fixation of sulfur dioxide into small organic molecules has attracted much attention. It will be highly desirable to find its application in organic synthesis, considering the enormous production of sulfur dioxide. Since Willis reported the first example through palladium-catalyzed aminosulfonylation by using DABCO-bis(sulfur dioxide) as the source of sulfur dioxide,1a continuous efforts have been underway.1–3 For instance, Wang and co-workers reported a copper(II)-catalyzed, three-component reaction of triethoxysilanes, sulfur dioxide, and hydrazines, leading to N-aminosulfonamides in good yields.2i Recently, we described an efficient route to N-aminosulfonamides via a three-component reaction of sulfur dioxide, aryldiazonium salt, and hydrazines.3 The transformation proceeded through a radical process under extremely mild conditions. Prompted by this result, we hypothesized that 2-alkynylaryldiazonium tetrafluoroborate 1 could be utilized in the reaction of sulfur dioxide with hydrazine as well. We envisioned that an intramolecular 6-endo cyclization would happen to afford 2H-benzo[e][1,2]thiazine 1,1-dioxide A4 if a suitable metal catalyst was presented in the reaction system (Scheme 1, eqn (1)). This tandem approach seemed feasible, which combined the insertion of sulfur dioxide and metal-catalyzed 6-endo cyclization in a one-pot procedure.
 | ||
| Scheme 1 Proposed three-component reaction of 2-alkynylaryldiazonium tetrafluoroborate, sulfur dioxide, with hydrazine. | ||
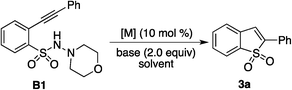
Due to the importance of benzo[e][1,2]thiazine 1,1-dioxide (compound A, Scheme 1) in many natural products and pharmaceuticals (e.g., ampiroxicam, an anti-inflammatory and analgesic drug A),5 we started to explore the possibility of this three-component reaction of 2-alkynylaryldiazonium tetrafluoroborate 1, sulfur dioxide and hydrazine. Initial studies were performed by using 2-alkynylphenyldiazonium tetrafluoroborate 1a, DABCO-bis(sulfur dioxide) 2, and morpholin-4-amine as the starting materials (Scheme 1, eqn (2)). Only N-morpholino-2-(phenylethynyl)benzenesulfonamide B1 was generated in the absence of a metal catalyst. To our surprise, we did not observe the formation of the desired benzo[e][1,2]thiazine 1,1-dioxide A1 under palladium catalysis. Instead, a small amount of benzo[b]thiophene 1,1-dioxide 3a was obtained. This result was interesting, since morpholin-4-amine was not incorporated in the final product and two new C–S bonds were formed. It is well known that benzo[b]thiophene 1,1-dioxide derivatives can behave as an absorbance and fluorescence switch, leading to a multi-addressable system when triggered by ions, protons and light.6 Additionally, they have been found as inhibitors of hepatitis C virus NS5B polymerase as well as carbonic anhydrase inhibitors of cytosolic/tumor-associated carbonic anhydrase isozymes.7 Moreover, complex molecules could be obtained using benzo[b]thiophene 1,1-dioxides as the starting materials.8 Thus, considering the importance of the benzo[b]thiophene 1,1-dioxide scaffold in material science, medicinal chemistry and organic synthesis, this novel observation encouraged us for its further exploration.
Since N-morpholino-2-(phenylethynyl)benzenesulfonamide B1 was obtained in the absence of a metal catalyst, we wondered whether benzo[b]thiophene 1,1-dioxide 3a was produced from the reaction of N-morpholino-2-(phenylethynyl)benzene-sulfonamide B1. Therefore, the reactions of N-morpholino-2-(phenylethynyl)benzenesulfonamide B1 catalyzed by palladium acetate in acetonitrile at 80 °C were examined. The results are presented in Table 1. It was found that the presence of base affected the final outcome. No reaction occurred when t-BuOK acted as the base in the reaction system (Table 1, entry 1). A trace amount of product 3a was detected when cesium carbonate was used as a replacement (Table 1, entry 2). Gratifyingly, benzo[b]thiophene 1,1-dioxide 3a was isolated and obtained in 37% yield when the base was changed to potassium carbonate (Table 1, entry 3). Further screening of bases revealed that the reaction worked efficiently in the presence of sodium acetate, giving rise to the expected product 3a in 60% yield (Table 1, entries 4–10). We subsequently examined the reaction in different solvents (Table 1, entries 11–15). Unfortunately, no better results were obtained. Therefore, we shifted our focus to other metal catalysts, such as rhodium and copper salts. The yield was improved when copper(I) bromide was used as the catalyst, providing the desired product 3a in 70% yield (Table 1, entry 25). A similar result was obtained when the reaction was performed at a higher temperature (Table 1, entry 26). However, at 50 °C, no reaction occurred (Table 1, entry 27). The yield could increase to 80% when 3.0 equiv. of base was added to the reaction (Table 1, entry 28). An inferior result was obtained when the amount of copper(I) bromide decreased (data not shown in Table 1).
| Entry | [M] | Base | Solvent | Yielda (%) |
|---|---|---|---|---|
| a Isolated yield based on N-morpholino-2-(phenylethynyl)benzene-sulfonamide B1. b The reaction was performed at 90 °C. c The reaction occurred at 50 °C. d In the presence of 3.0 equiv. of base. | ||||
| 1 | Pd(OAc)2 | t-BuOK | MeCN | nr |
| 2 | Pd(OAc)2 | Cs2CO3 | MeCN | Trace |
| 3 | Pd(OAc)2 | K2CO3 | MeCN | 37 |
| 4 | Pd(OAc)2 | Na2CO3 | MeCN | 41 |
| 5 | Pd(OAc)2 | K3PO4 | MeCN | 17 |
| 6 | Pd(OAc)2 | KHCO3 | MeCN | 33 |
| 7 | Pd(OAc)2 | NaHCO3 | MeCN | 36 |
| 8 | Pd(OAc)2 | DABCO | MeCN | 29 |
| 9 | Pd(OAc)2 | KOAc | MeCN | 43 |
| 10 | Pd(OAc)2 | NaOAc | MeCN | 60 |
| 11 | Pd(OAc)2 | NaOAc | Toluene | 12 |
| 12 | Pd(OAc)2 | NaOAc | 1,4-Dioxane | 15 |
| 13 | Pd(OAc)2 | NaOAc | AmylOH | 41 |
| 14 | Pd(OAc)2 | NaOAc | DMSO | 40 |
| 15 | Pd(OAc)2 | NaOAc | DMF | 56 |
| 16 | PdCl2 | NaOAc | MeCN | 28 |
| 17 | Pd(TFA)2 | NaOAc | MeCN | 17 |
| 18 | [Rh(COD)Cl]2 | NaOAc | MeCN | 25 |
| 19 | Cu(OAc)2 | NaOAc | MeCN | 54 |
| 20 | Cu(OTf)2 | NaOAc | MeCN | 43 |
| 21 | CuI | NaOAc | MeCN | 39 |
| 22 | CuCl2 | NaOAc | MeCN | 62 |
| 23 | CuBr2 | NaOAc | MeCN | 58 |
| 24 | CuCl | NaOAc | MeCN | 64 |
| 25 | CuBr | NaOAc | MeCN | 70 |
| 26b | CuBr | NaOAc | MeCN | 68 |
| 27c | CuBr | NaOAc | MeCN | nr |
| 28d | CuBr | NaOAc | MeCN | 80 |
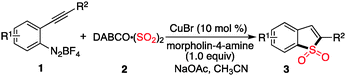
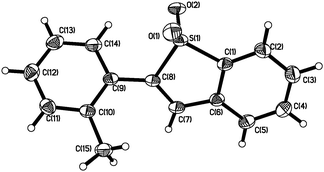
With the above results in hand, we re-investigated the reaction of 2-alkynylphenyldiazonium tetrafluoroborate 1a with DABCO-bis(sulfur dioxide) 2 in the presence of copper(I) bromide (10 mol%), morpholin-4-amine (1.0 equiv.) and NaOAc (3.0 equiv.) in MeCN. As expected, the corresponding product 3a was generated in 65% yield. A control experiment revealed that no reaction occurred in the absence of morpholin-4-amine, which demonstrated that the reaction proceeded through the intermediate of N-morpholino-2-(phenylethynyl)benzenesulfonamide B1. Moreover, other hydrazines were examined in the reaction, such as 1-methyl-1-phenylhydrazine, 1-ethyl-1-phenylhydrazine, 1,1-diphenylhydrazine. However, the yields were lower (37–40%). Therefore, the scope generality of this insertion of sulfur dioxide was then explored. The results are shown in Table 2. We found that all reactions took place smoothly under the optimized conditions to afford the desired benzo[b]thiophene 1,1-dioxides 3 in moderate to good yields. Various functional groups (such as fluoro, chloro, methyl, and ester) were compatible. The thiophenyl-substituted product 3n could be produced in 54% yield as well under standard conditions. In the meantime, the structure of compound 3i was unambiguously identified via X-ray crystallography analysis (Fig. 1).
| a Isolated yield based on DABCO-bis(sulfur dioxide) 2. |
|---|
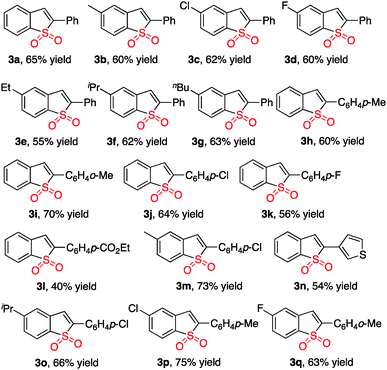
|
It was reported that metal sulfinates could be easily generated from N-aminosulfonamide derivatives in the presence of a suitable base.9 Thus, the formation of benzo[b]thiophene 1,1-dioxide was reasonable, although the result was not expected initially, since the N-aminosulfonamide would serve as a masked sulfinate. The presence of a metal catalyst would activate the triple bond in the substrate, which in turn would facilitate the intramolecular cyclization of sulfinate (generated in situ) to alkyne. Cleavage of the S–N bond in N-aminosulfonamide in the presence of a base at high temperature was also observed by Willis and Toste.1c,2c Based on the experimental results and previous reports as mentioned above, a possible mechanism was proposed (Scheme 2). We reasoned that 2-alkynylphenyldiazonium tetrafluoroborate 1 would react with DABCO-bis(sulfur dioxide) 2 and morpholin-4-amine, leading to N-morpholino-2-(phenylethynyl)benzenesulfonamide B. Sodium sulfinate C would be formed in the presence of base, with the cleavage of the S–N bond of N-aminosulfonamide. Subsequently, a metal-catalyzed intramolecular 5-endo cyclization followed by protonation would produce the corresponding product 3.
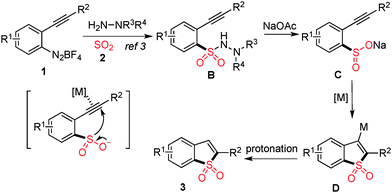 | ||
| Scheme 2 Possible mechanism for reaction of 2-alkynylaryldiazonium tetrafluoroborate with sulfur dioxide. | ||
In conclusion, we developed a copper(I)-catalyzed reaction of 2-alkynylaryldiazonium tetrafluoroborate with sulfur dioxide. The transformation involving the insertion of sulfur dioxide and intramolecular 5-endo cyclization proceeds efficiently to generate benzo[b]thiophene 1,1-dioxides in moderate to good yields. During the process, two new C–S bonds were formed. Exploration of the insertion of sulfur dioxide for the synthesis of other heterocycles is ongoing in our laboratory.
Financial support from the National Natural Science Foundation of China (21172038, 21372046) is gratefully acknowledged.
Notes and references
- (a) B. Nguyen, E. J. Emmet and M. C. Willis, J. Am. Chem. Soc., 2010, 132, 16372 CrossRef CAS PubMed; (b) A. J. Emmet, C. S. Richards-Taylor, B. Nguyen, A. B. Garcia-Rubia, R. Hayter and M. C. Willis, Org. Biomol. Chem., 2012, 10, 4007 RSC; (c) C. S. Richards-Taylor, D. C. Blakemore and M. C. Willis, Chem. Sci., 2014, 5, 222 RSC; (d) E. J. Emmett, B. R. Hayter and M. C. Willis, Angew. Chem., Int. Ed., 2013, 52, 12679 CrossRef CAS PubMed; (e) A. S. Deeming, C. J. Rusell, A. J. Hennessy and M. C. Willis, Org. Lett., 2014, 16, 150 CrossRef CAS PubMed.
- (a) S. Ye and J. Wu, Chem. Commun., 2012, 48, 7753 RSC; (b) S. Ye and J. Wu, Chem. Commun., 2012, 48, 10037 RSC; (c) M. W. Johnson, S. W. Bagley, N. P. Mankad, R. G. Bergman, V. Mascitti and F. D. Toste, Angew. Chem., Int. Ed., 2014, 53, 4404 CrossRef CAS PubMed; (d) P. Bisseret and N. Blanchard, Org. Biomol. Chem., 2013, 11, 5393 RSC; (e) L. Martial, Synlett, 2013, 1595 Search PubMed; (f) W. Li, H. Li, P. Langer, M. Beller and X.-F. Wu, Eur. J. Org. Chem., 2014, 3101 CrossRef; (g) D. Zheng, Y. Li, Y. An and J. Wu, Chem. Commun., 2014, 50, 8886 RSC; (h) B. N. Rocke, K. B. Bahnck, M. Herr, S. Lavergne, V. Mascitti, C. Perreault, J. Polivkova and A. Shavnya, Org. Lett., 2014, 16, 154 CrossRef CAS PubMed; (i) X. Wang, L. Xue and Z. Wang, Org. Lett., 2014, 16, 4056 CrossRef CAS PubMed; (j) S. Ye, H. Wang, Q. Xiao, Q. Ding and J. Wu, Adv. Synth. Catal., 2014, 356, 3225 CrossRef CAS; (k) Y. An, D. Zheng and J. Wu, Chem. Commun., 2014, 50, 11746 RSC; (l) E. J. Emmett, B. R. Hayter and M. C. Willis, Angew. Chem., Int. Ed., 2014, 53, 10204 CrossRef CAS PubMed; (m) W. Li, M. Beller and X.-F. Wu, Chem. Commun., 2014, 50, 9513 RSC.
- D. Zheng, Y. An, Z. Li and J. Wu, Angew. Chem., Int. Ed., 2014, 53, 2451 CrossRef CAS PubMed.
- Q. Xiao, J. Sheng, Z. Chen and J. Wu, Chem. Commun., 2013, 49, 8647 RSC.
- (a) C. Valent, R. C. Guedes, R. Moreia, J. Iley, J. Gut and P. J. Rosenthal, Bioorg. Med. Chem. Lett., 2006, 16, 4115 CrossRef PubMed; (b) L. Zhuang, J. S. Wai, M. W. Embrey, T. E. Fisher, M. S. Egbertson, L. S. Payne, J. P. Guare, J. P. Vacca, D. J. Hazuda, P. J. Felock, A. L. Wolfe, K. A. Stilmock, M. V. Witmer, G. Moyer, W. A. Schleif, L. J. Gabryelski, Y. M. Leonard, J. J. Lynch, S. R. Michelson and S. D. Young, J. Med. Chem., 2003, 46, 453 CrossRef CAS PubMed; (c) Y. Misu and H. Togo, Org. Biomol. Chem., 2003, 11, 1342 RSC; (d) K. H. Ahn, S. K. Kim and C. Ham, Tetrahedron Lett., 1998, 39, 6321 CrossRef CAS; (e) G. J. Wells, M. Tao, K. A. Josef and R. Bihovsky, J. Med. Chem., 2001, 44, 3488 CrossRef CAS PubMed.
- (a) S. Chen, Y. Yang, Y. Wu, H. Tian and W. Zhu, J. Mater. Chem., 2012, 22, 5486 RSC; (b) S. Chen, L.-J. Chen, H.-B. Yang, H. Tian and W. Zhu, J. Am. Chem. Soc., 2012, 134, 13596 CrossRef CAS PubMed.
- (a) S. H. Kim, M. T. Tran, F. Ruebsam, A. X. Xiang, B. Ayida, H. McGuire, D. Ellis, J. Blazel, C. V. Tran and D. E. Murphy, Bioorg. Med. Chem. Lett., 2008, 18, 4181 CrossRef CAS PubMed; (b) A. Innocenti, R. Villar, V. Martinez-Merino, M. J. Gil, A. Scozzafava, D. Vullo and C. T. Supuran, Bioorg. Med. Chem. Lett., 2005, 15, 4872 CrossRef CAS PubMed; (c) R. Villar, I. Encio, M. Migliaccio, M. J. Gil and V. Martinez-Merino, Bioorg. Med. Chem., 2004, 12, 963 CrossRef CAS PubMed.
- (a) N. V. Lakshmi, P. Thirumurugan, C. Jayakumar and P. T. Perumal, Synlett, 2010, 955 CAS; (b) N. Malatesti, A. N. Boa, S. Clark and R. Westwood, Tetrahedron Lett., 2006, 47, 5139 CrossRef CAS PubMed; (c) K. Bougrin, M. Soufiaoui, A. Loupy and P. Jacquault, New J. Chem., 1995, 19, 213 CAS.
- (a) B. F. Powell, C. G. Overberger and J. P. Anselme, J. Heterocycl. Chem., 1983, 20, 121 CrossRef CAS; (b) P. Carter and T. S. Stevens, J. Chem. Soc., 1961, 1743 RSC; (c) D. M. Lemal, T. W. Rave and S. D. McGregor, J. Am. Chem. Soc., 1963, 85, 1944 CrossRef CAS; (d) D. M. Lemal, F. Menger and E. Coats, J. Am. Chem. Soc., 1964, 86, 2395 CrossRef CAS; (e) L. A. Carpino, Chem. Ind., 1957, 172 CAS; (f) L. A. Carpino, J. Am. Chem. Soc., 1957, 79, 4427 CrossRef CAS; (g) W. Baker, J. F. W. McOmie and D. R. Preston, J. Chem. Soc., 1961, 2971 RSC; (h) A. Dornow and W. Bartsch, Liebigs Ann. Chem., 1957, 602, 23 CrossRef CAS; (i) R. Ballini, E. Marcantoni and M. Petrini, Tetrahedron, 1989, 45, 6791 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedure, characterization data, CIF file of compound 3i, 1H and 13C NMR spectra of compounds 3. CCDC 1011150. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4cc07938h |
| ‡ Y. Luo and X. Pan contributed equally. |
| This journal is © The Royal Society of Chemistry 2015 |