Enantioselective synthesis of arylglycine derivatives by direct C–H oxidative cross-coupling†
Abstract
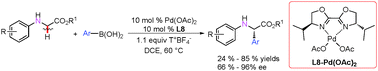
A new method for the synthesis of chiral α-amino acid derivatives by enantioselective C–H arylation of N-aryl glycine esters with aryl boric acids in the presence of a chiral Pd(II)-catalyst has been developed. This work successfully integrates the direct C–H oxidation with asymmetric arylation and exhibits excellent enantioselectivity.

 Please wait while we load your content...
Please wait while we load your content...