Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Reversible activation of pH-sensitive cell penetrating peptides attached to gold surfaces†
Joe E.
Baio‡§
a,
Denise
Schach‡¶
a,
Adrian V.
Fuchs
a,
Lars
Schmüser
a,
Nils
Billecke
a,
Christoph
Bubeck
a,
Katharina
Landfester
a,
Mischa
Bonn
a,
Michael
Bruns
b,
Clemens K.
Weiss
ac and
Tobias
Weidner
*a
aMax Planck Institute for Polymer Research, 55270 Mainz, Germany. E-mail: weidner@mpip-mainz.mpg.de
bKarlsruhe Institute of Technology, Institute for Applied Materials and Karlsruhe Nano Micro Facility, 76344 Eggenstein-Leopoldshafen, Germany
cUniversity of Applies Sciences Bingen, 55411 Bingen, Germany
First published on 6th October 2014
Abstract
pH-sensitive viral fusion protein mimics are widely touted as a promising route towards site-specific delivery of therapeutic compounds across lipid membranes. Here, we demonstrate that a fusion protein mimic, designed to achieve a reversible, pH-driven helix-coil transition mechanism, retains its functionality when covalently bound to a surface.
The controlled and selective delivery of therapeutic compounds into cells is a key element of targeted drug delivery therapies. Promising targeting mechanisms include drug loaded nanoparticles (NPs). The surface functionalities of NPs can be designed to control particle uptake in cells.1–11 Therefore, to target specific cell types or tissues, targeting ligands, such as folic acid,12–15 carbohydrates,16,17 peptides or proteins14,18–21 can be attached to a particle's surface.6,22,23 However, one hurdle that has impeded the widespread deployment of drug loaded NPs is the fact that certain drugs are only active within a cell's cytosol. Following cellular uptake, NPs are usually trapped in endosomes,5 unable to reach their therapeutic targets. To reach the cytosol, NPs need the ability to penetrate through the endosomal membrane barrier while ideally leaving the cell plasma membrane intact.
Viruses face these same challenges when delivering their genes from endosomes into the cytosol and have devised specialized fusion proteins that promote endosomal escape.24 The activity of these proteins is triggered by a structural transition induced by the low pH in endosomes (pH ∼ 5); this transition is reversed within the cell cytosol (pH ∼ 7), de-activating the protein. Artificial peptide mimics of these pH-sensitive viral fusion proteins therefore, represent a promising route to achieve site-specific membrane interactions.1–4,25–29 A member of the family of pH-sensitive peptides, GALA (WEAALAEAL-AEALAEHLAEALAEALEALAA), has attracted particular attention as a potential candidate for effective and specific permeation.2 GALA's secondary structure depends on the pH of the local environment. In physiologically low pHs, i.e. pH = 5, GALA forms a stable α-helical secondary structure. Due to the hydrophobic/hydrophilic surfaces in this state, GALA is likely to assemble into bundles of helices, causing pore formation and membrane leakage. At basic pH, the glutamic acid side chains are deprotonated and, as a result, charged, causing the helix to destabilize. In this high-pH state GALA is no longer membrane active.26
GALA has been shown to effectively penetrate and permeate cell lipid bilayers and enhance the endosomal escape following internalization of drug-loaded vesicles via endocytosis, and, thus, the drug delivery efficiency.26,30 However, the application of GALA bound to surfaces has not been reported so far. For targeted drug delivery and the design of ‘smart’ biological surfaces, the peptide needs to be immobilized onto a substrate. The secondary structure can be strongly affected by charge and hydrophobicity of surfaces and the confinement in the high peptide concentration near the surface may interfere with pH-controlled folding and unfolding and might alter the pK-value.31–33 Therefore, the challenge with the functionality of solid-supported proteins and self-assembled monolayers (SAM) of peptides is understanding the interaction between the protein and surface.
A barrier for the rational design of efficient and reliable drug delivery vehicles by bioengineers and chemists is our current lack of understanding of structural properties of such pH sensitive peptide-SAMs on a molecular level. Surface sensitive spectroscopies have recently been used to determine folding and orientation of a range of peptides covalently attached to surfaces for applications in catalysis,34 biomaterials35 and antimicrobial surfaces.36 Progress in the field has also been summarized in recent reviews about peptide SAMs.37,38 In this work, we approximate the conditions of a GALA-SAM bound to gold NPs by specific binding of GALA onto gold surfaces via a cysteine residue we have synthetically added at the C-terminus of the GALA sequence (GALA-Cys: WEAALAEALAEALAEHLAEALAEALEALAA-C). Cysteine side chains can reliably link peptides or proteins to gold.39 The covalent thiol–gold bond induces ordered adsorption and can lead to well-aligned protein films.39–42 We verified that the addition of a cysteine residue does not interfere with the pH-driven refolding by infrared spectra of GALA-Cys dissolved in bulk D2O (ESI†). A schematic of the GALA-Au binding scheme is shown in Fig. 1. The formation of a closed, well-defined protein monolayer is crucial, therefore, quantitative characterization of the composition and chemical integrity of GALA-Cys films on Au were provided by X-ray photoelectron spectroscopy (XPS), surface plasmon resonance (SPR), electrochemical impedance spectroscopy (EIS), surface-enhanced infrared absorption spectroscopy (SEIRAS) and atomic force microscopy (AFM). Resistance and capacitance values collected across the GALA-Cys film by EIS indicate a homogenous film which is also supported by atomic force microscopy (AFM) images recorded before and after the SAM-formation that illustrate the lack of aggregates, particles or domains at the surface (see ESI†). The XPS determined film compositions are in agreement with the theoretical composition of a GALA-cys monolayer on Au (see ESI†). The thickness of this adsorbed protein layer, determined by SEIRAS and SPR angle scans taken before and after monolayer formation, yields a peptide layer thickness of 1.5 nm ± 0.5 nm (see ESI†). This thickness value indicates an inclined adsorption geometry. The tilt angle of the peptide helix can be estimated to be ∼20° with respect to the surface assuming a peptide length of ∼5 nm. At a 20° angle, a GALA peptide occupies an area of about 450 Å2, compared with a footprint of ∼100 Å2 expected for a fully upright orientation. Combined, the surface analytical techniques illustrated a well packed and strongly inclined monolayer of GALA-Cys bound covalently to the gold surface.
To determine if the secondary structure of GALA remains pH-sensitive after surface attachment we probed a monolayer of the GALA-Cys peptides adsorbed onto a gold film with sum frequency generation (SFG) vibrational spectroscopy. Like other vibrational spectroscopic techniques, amide modes observed within SFG spectra can be used to identify secondary structures. However, SFG selection rules dictate that an SFG response will only originate from a surface or interface where inversion symmetry is broken. As a result of these selection rules – we expect that any vibrational mode observed within the amide I stretching region will only originate from ordered, non-antiparallel or non-random secondary structures immobilized at the Au substrate.
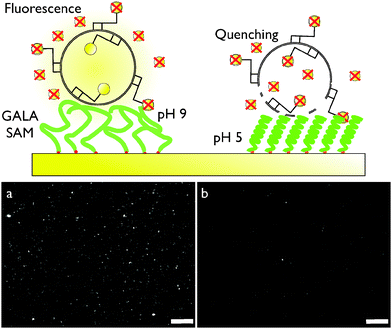
GALA-Cys was self-assembled on a 7 nm gold island film evaporated onto a CaF2 prism (Fig. 2, upper scheme). SFG spectra in the amide I region of the vibrational spectrum (1550–1750 cm−1) were collected at four consecutive different buffer pH values (pH = 5, 9, 12 and 3) (Fig. 2, lower scheme). At pH = 5, the spectrum contains a vibrational mode at 1650 cm−1 which is characteristic of ordered α-helices, and two weaker bands at 1630 and 1675 cm−1 related to ordered β-sheets. As the pH was increased to 9 and then to 12 the magnitude of the 1650 cm−1 peak is dramatically reduced, while the two other modes related to β-sheets are small but remain. Random and unordered secondary structure may also exist at this pH but are not detected by SFG. As the pH of the buffer solution is lowered down to pH 3, the peak at 1650 cm−1 reappears, consistent with the notion that the pH triggered, reversible conformational change into α-helices is retained when GALA is immobilized on surfaces. To test whether surface-bound GALA can disrupt lipid bilayers in a similar way as free GALA in solution, a fluorescence imaging assay was performed (Fig. 3). In analogy to leakage studies in solution, rhodamine-B labeled dipalmitoylphosphatidylcholine (DPPC) vesicles where deposited onto a GALA-Cys SAM in the presence of sodium dithionite (DTT) solution. Dye molecules attached to the outer leaflet were oxidized and quenched by DDT – rhodamine attached to the inner leaflet was protected by the vesicle membrane. Fig. 3a and b show fluorescence images recorded at pH 9 and pH 5. At high pH the fluorescence of intact individual and clustered vesicles are clearly visible. At pH 5 the fluorescence yield is greatly reduced by quenching of rhodamine at the inner leaflet due to membrane leakage caused by active GALA peptides.
 | ||
| Fig. 2 Upper scheme: experimental SFG setup. Lower scheme: SFG spectra of GALA adsorbed on Au (black) measured under acidic, basic, and again acidic conditions. The spectra were taken under PPP-polarization conditions and Lorentzian band shapes were fitted to the bands (red) – see ESI† for fitting details. | ||
While detailed cell studies are needed to investigate the impact, for example, of GALA on particle uptake and endosomal escape under physiological conditions, this investigation takes an important first step. The results illustrate that the controlled confinement of viral fusion peptide mimics to a gold surface does not interfere with the controlled folding and membrane activity of the GALA sequence. We have demonstrated that a GALA-Cys SAM can be self-assembled onto Au-surfaces by inserting a single cysteine to the peptide terminus. Both the addition of a single Cys and chemical adsorption onto a solid substrate does not alter the pH-driven structural transition of GALA or its activity – indicating that the attachment of GALA to surfaces is a viable approach to functionalizing particles surfaces with pH responsive peptide coatings.
T.W., J.E.B. and D.S. thank the Deutsche Forschungsgemeinschaft (WE 4478/2-1) and European Union Marie Curie Program for support of this work (CIG grant #322124). J.E.B. thanks the National Science Foundation for a research fellowship (NSF grant #1202620). This work is part of the research program of the Max Planck Society. We thank Gunnar Glaser for recording SEM images, Beate Müller for HPLC analysis, Walter Scholdei for the construction of the ATR cell and FTIR support, and Sapun Parekh for providing fluorescence imaging equipment.
Notes and references
- J. Andrieu, N. Kotman, M. Maier, V. Mailander, W. S. Strauss, C. K. Weiss and K. Landfester, Macromol. Rapid Commun., 2012, 33, 248–253 CrossRef CAS PubMed.
- D. Bartczak, O. L. Muskens, S. Nitti, T. Sanchez-Elsner, T. M. Millar and A. G. Kanaras, Small, 2012, 8, 122–130 CrossRef CAS PubMed.
- J. Dausend, A. Musyanovych, M. Dass, P. Walther, H. Schrezenmeier, K. Landfester and V. Mailander, Macromol. Biosci., 2008, 8, 1135–1143 CrossRef CAS PubMed.
- S. Lorenz, C. P. Hauser, B. Autenrieth, C. K. Weiss, K. Landfester and V. Mailander, Macromol. Biosci., 2010, 10, 1034–1042 CrossRef CAS PubMed.
- V. Mailänder and K. Landfester, Biomacromolecules, 2009, 10, 2379–2400 CrossRef PubMed.
- J. Nicolas, S. Mura, D. Brambilla, N. Mackiewicz and P. Couvreur, Chem. Soc. Rev., 2013, 42, 1147–1235 RSC.
- C. K. Weiss, M. V. Kohnle, K. Landfester, T. Hauk, D. Fischer, J. Schmitz-Wienke and V. Mailander, ChemMedChem, 2008, 3, 1395–1403 CrossRef CAS PubMed.
- M. R. Lorenz, V. Holzapfel, A. Musyanovych, K. Nothelfer, P. Walther, H. Frank, K. Landfester, H. Schrezenmeier and V. Mailander, Biomaterials, 2006, 27, 2820–2828 CrossRef CAS PubMed.
- C. K. Weiss, M. R. Lorenz, K. Landfester and V. Mailander, Macromol. Biosci., 2007, 7, 883–896 CrossRef CAS PubMed.
- M. Zhu, G. Nie, H. Meng, T. Xia, A. Nel and Y. Zhao, Acc. Chem. Res., 2013, 46, 622–631 CrossRef CAS PubMed.
- L. Florez, C. Herrmann, J. M. Cramer, C. P. Hauser, K. Koynov, K. Landfester, D. Crespy and V. Mailänder, Small, 2012, 8, 2222–2230 CrossRef CAS PubMed.
- S. U. Frick, N. Bacher, G. Baier, V. Mailander, K. Landfester and K. Steinbrink, Macromol. Biosci., 2012, 12, 1637–1647 CrossRef CAS PubMed.
- Y. Lu and P. S. Low, Adv. Drug Delivery Rev., 2012, 64, 342–352 CrossRef PubMed.
- S. Franzen, Expert Opin. Drug Delivery, 2011, 8, 281–298 CrossRef CAS PubMed.
- Q. Tu, Y. Zhang, R. Liu, J.-C. Wang, L. Li, N. Nie, A. Liu, L. Wang, W. Liu, L. Ren, X. Wang and J. Wang, Curr. Med. Chem., 2012, 19, 3152–3162 CrossRef CAS.
- R. A. Dwek, Chem. Rev., 1996, 96, 683–720 CrossRef CAS PubMed.
- K. L. White, T. Rades, R. H. Furneaux, P. C. Tyler and S. Hook, J. Pharm. Pharmacol., 2006, 58, 729–737 CrossRef CAS PubMed.
- I. A. Khalil, K. Kogure, S. Futaki, S. Hama, H. Akita, M. Ueno, H. Kishida, M. Kudoh, Y. Mishina, K. Kataoka, M. Yamada and H. Harashima, Gene Ther., 2007, 14, 682–689 CrossRef CAS PubMed.
- J. H. Lee, J. A. Engler, J. F. Collawn and B. A. Moore, FEBS, 2001, 268, 2004–2012 CAS.
- R. Qiao, Q. Jia, S. Hüwel, R. Xia, T. Liu, F. Gao, H.-J. Galla and M. Ga, ACS Nano, 2012, 6, 3304–3310 CrossRef CAS PubMed.
- S. Santra, H. Yang, D. Dutta, J. T. Stanley, P. H. Holloway, W. Tan, B. M. Moudgil and R. A. Mericle, Chem. Commun., 2004, 2810–2811 RSC.
- B. L. Droumaguet, J. Nicolas, D. Brambilla, S. Mura, A. Maksimenko, L. D. Kimpe, E. Salvati, C. Zona, C. Airoldi, M. Canovi, M. Gobbi, M. Noiray, B. L. Ferla, F. Nicotra, W. Scheper, O. Flores, M. Masserini, K. Andrieux and P. Couvreur, ACS Nano, 2012, 6, 5866–5879 CrossRef PubMed.
- K. Landfester and V. Mailander, Expert Opin. Drug Delivery, 2013, 10, 593–609 CrossRef CAS PubMed.
- K. Narayanan, S. K. Yen, Q. Dou, P. Padmanabhan, T. Sudhaharan, S. Ahmed, J. Y. Ying and S. T. Selvan, Sci. Rep., 2013, 3, 2184 Search PubMed.
- O. A. Andreev, D. M. Engelman and Y. K. Reshetnyak, Mol. Membr. Biol., 2010, 27, 341–352 CrossRef CAS PubMed.
- W. Li, F. Nicol and F. C. Szoka, Jr., Adv. Drug Delivery Rev., 2004, 56, 967–985 CrossRef CAS PubMed.
- R. A. Parente, S. Nir and F. C. Szoka, J. Biol. Chem., 1988, 263, 4724–4730 CAS.
- L. Yao, J. Daniels, A. Moshnikova, S. Kuznetsov, A. Ahmed, D. M. Engelman, Y. K. Reshetnyak and O. A. Andreev, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 465–470 CrossRef CAS PubMed.
- M. Zoonens, Y. K. Reshetnyak and D. M. Engelman, Biophys. J., 2008, 95, 225–235 CrossRef CAS PubMed.
- B. F. Lin, D. Missirlis, D. V. Krogstad and M. Tirrell, Biochemistry, 2012, 51, 4658–4668 CrossRef CAS PubMed.
- B. Sivaraman, K. P. Fears and R. A. Latour, Langmuir, 2009, 25, 3050–3056 CrossRef CAS PubMed.
- J. Buijs, P. A. W. vandenBerg, J. W. T. Lichtenbelt, W. Norde and J. Lyklema, J. Colloid Interface Sci., 1996, 178, 594–605 CrossRef CAS.
- D. G. Castner and B. D. Ratner, Surf. Sci., 2002, 500, 28–60 CrossRef CAS.
- E. Gross, J. H. Liu, S. Alayoglu, M. A. Marcus, S. C. Fakra, F. D. Toste and G. A. Somorjai, J. Am. Chem. Soc., 2013, 135, 3881–3886 CrossRef CAS PubMed.
- J. M. Kaplan, J. Shang, P. Gobbo, S. Antonello, L. Armelao, V. Chatare, D. M. Ratner, R. B. Andrade and F. Maran, Langmuir, 2013, 29, 8187–8192 CrossRef CAS PubMed.
- Z. Wang, X. Han, N. He, Z. Chen and C. L. Brooks, 3rd, J. Phys. Chem. B, 2014, 118, 5670–5680 CrossRef CAS PubMed.
- S. A. Onaizi and S. S. Leong, Biotechnol. Adv., 2011, 29, 67–74 CrossRef CAS PubMed.
- F. Costa, I. F. Carvalho, R. C. Montelaro, P. Gomes and M. C. Martins, Acta Biomater., 2011, 7, 1431–1440 CrossRef CAS PubMed.
- S. L. Dawson and D. A. Tirrell, J. Mol. Recognit., 1997, 10, 18–25 CrossRef CAS.
- J. E. Baio, T. Weidner and D. G. Castner, Proteins at Interfaces III, American Chemical Society, 2012, vol. 1120, pp. 761–779 Search PubMed.
- L. Baugh, T. Weidner, J. E. Baio, P.-C. T. Nguyen, L. J. Gamble, P. S. Stayton and D. G. Castner, Langmuir, 2010, 26, 16434–16441 CrossRef CAS PubMed.
- R. G. Nuzzo and D. L. Allara, J. Am. Chem. Soc., 1983, 105, 4481–4483 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Sample preparation, surface characterization. See DOI: 10.1039/c4cc07278b |
| ‡ JEB and DS contributed to this work equally. |
| § Current address: Oregon State University, Corvallis, OR 97331, USA. |
| ¶ Current address: University of Chicago, Chicago, IL 60637, USA. |
| This journal is © The Royal Society of Chemistry 2015 |