Open Access Article
Open Access ArticleTitania photocatalysis through two-photon band-gap excitation with built-in rhodium redox mediator†
Joanna
Kuncewicz
*a and
Bunsho
Ohtani
b
aFaculty of Chemistry, Jagiellonian University, ul. R. Ingardena 3, 30-011 Kraków, Poland. E-mail: kuncewic@chemia.uj.edu.pl; Fax: +48 126340515; Tel: +48 126632005
bCatalysis Research Center, Hokkaido University, Sapporo 001-0021, Japan. E-mail: ohtani@cat.hokudai.ac.jp; Fax: +81 11 706 9133; Tel: +81 11 706 9132
First published on 31st October 2014
Abstract
Titania particles modified with an extremely small amount (<0.01 mol%) of a rhodium species exhibited photocatalytic activity for the oxidative decomposition of acetaldehyde in air under visible-light irradiation. The reaction proceeded via a two-photon band-gap excitation mechanism resembling the Z-scheme with an external redox couple, using a built-in Rh(III)–Rh(IV) redox couple.
It has been often reported in studies of visible-light-active photocatalysts that titanium(IV) oxide (titania) has many advantages as a photocatalyst, including chemical stability both in the dark and under photoirradiation, sufficient redox ability in the excited state that can induce water splitting, non-toxicity, and high availability. However, titania can only be excited by UV light.1 To overcome this sole disadvantage of titania, there are two strategies. One is to find a photocatalyst that absorbs visible light with the advantages of titania, although such a “superman” metal–oxide photocatalyst has not yet been discovered despite 40 years of research. Another strategy is to modify titania to give visible-light absorption by doping with nitrogen,2 sulfur3 or carbon,4 or by loading metal oxide clusters5 to obtain filled and vacant electronic states, respectively, in the band gap of titania. However, the redox ability of titania may be lost, because in these strategies, positive holes in the valence band (VB) and photoexcited electrons in the conduction band (CB) of titania, which induce redox reactions when titania is excited by UV light, cannot be used. Therefore, a further strategy for developing visible-light-active titania is to introduce a mechanism of two-photon band-gap excitation.
Rhodium (Rh)-doping, where base material is modified with a small amount of a Rh species without changing the original crystal structure, has been used to develop visible-light-active titania6–9 or strontium titanate (SrTiO3).10–14 It was proposed that photoexcitation from Rh(III) to the CB of titania or SrTiO3 leaves Rh(IV), which oxidizes compounds adsorbed on the surface. Thus, although the CB electrons can be used in a reduction step, as in UV band-gap excitation, oxidation is induced by a localized species, Rh(IV), not by VB positive holes. Here we report the visible-light-induced photocatalysis by titania modified with an extremely small amount of Rh, in which both VB holes and CB electrons participate through two-photon band-gap excitation.
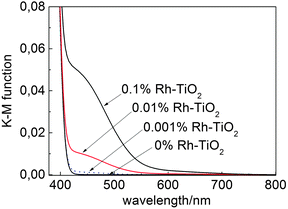
In the present work, Rh-modified titania (Rh–TiO2) samples were prepared by impregnating commercial titania (anatase; Showa Denko Ceramics FP6) with rhodium(III) chloride (RhCl3; Wako Pure Chemical; 0.001–0.1 mol%) followed by calcination in air at 923 K for 3 h to produce rutile materials, as confirmed by X-ray diffractometry (ESI†). A reference sample (0% Rh–TiO2; rutile) was prepared by a similar calcination process without adding RhCl3. Representative photoabsorption spectra are shown in Fig. 1. In addition to the absorption at <420 nm by rutile titania, two shoulders were present at 450 and 620 nm that were attributed to Rh(III) and Rh(IV), respectively,7,15 after Rh-modification. A peak at 620 nm was evident for the sample with a relatively large amount of Rh (>0.01%), what indicated that in studied samples loaded Rh was mainly Rh(III).
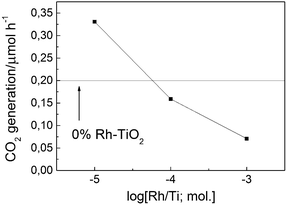
The photocatalytic activity of samples was evaluated by the rate of carbon dioxide (CO2) liberation by visible-light photoirradiation (>440 nm) of a sample (50 mg) in a 1 cm2 sample holder placed in a reaction chamber (357 mL) containing air and 224 ppm of acetaldehyde (AcH). As Fig. 2 shows, activity higher than that of bare TiO2 was observed for the sample modified with an extremely small amount of Rh (<0.01%). The origin of the visible-light activity of bare TiO2 may be a very small undetectable absorption arising from surface states and is still ambiguous; however, the dependence of the activity on the loading suggests that the TiO2 activity might be diminished by overlaying a larger amount of Rh species, which hinders the intrinsic photoabsorption of titania. A possible reason for the lower photocatalytic activity of samples with a larger amount of Rh may also be the higher content of Rh(IV), which was negligible for samples with an extremely low Rh loading (Fig. 1), which may act as an electron trap that enhances electron–hole recombination.7,12 Thus, an extremely small amount of Rh gives titania visible-light activity. The optimal Rh concentration should be around 0.0005–0.001%, which was also confirmed by studies of the photocatalytic activity of less active samples calcined at a slightly higher temperature (973 K) (ESI†). Characterization of the sample with Rh at such low concentrations was difficult because of the small intensity of the photoabsorption of the Rh species. Therefore, a 0.01% sample was used for the following experiments. The active Rh species were probably small particles attached to the surface because repeated rinsing of the samples with water caused appreciable, but not complete, loss of the photoabsorption and almost complete loss of the photocatalytic activity under visible-light irradiation. Because of the extremely small amount of Rh atoms (0.008–0.08) per 1 nm2 of titania with a specific surface area of 10 m2 g−1, isolated Rh ions may be dispersed on the titania surface in the form of oxide clusters. They may even be embedded in the titania crystal lattice near the surface, which is supported by the observed strong interaction of the Rh species with titania.
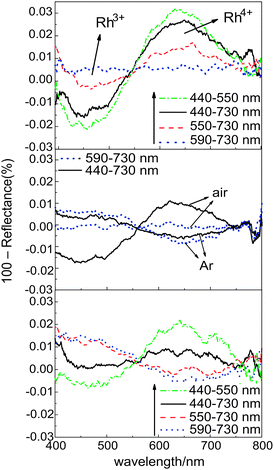
It should be noted that the photoabsorption of the active samples changed during the photoirradiation and the behavior depended on the photoirradiation wavelength range. Upon irradiation with light in the wavelength range of 440–550 nm, which overlapped the absorption band assigned to Rh(III), decrease in the intensity of that band was observed whereas the intensity of the band assigned to Rh(IV) increased. Subsequent irradiation with full wavelength range light (440–730 nm) caused a slight increase in the intensity of the 450 nm band and a decrease in the 620 nm band, respectively; the longer wavelength irradiation (550–730 nm) recovered Rh(III) from the Rh(IV) (Fig. 3, upper panel). Irradiation with longer wavelength light (590–730 nm) recovered Rh(III) to the original concentration, because the transition of Rh(III) to Rh(IV) cannot be induced by this longer-wavelength irradiation. Photoinduced regeneration of Rh(III) under the irradiation with light from the range 590–730 nm, appeared to be slower than the reverse process. The in situ photoabsorption measurements shown in Fig. 3 (lower panel) confirmed the light wavelength-controlled reversibility of these redox processes during the photocatalytic reactions.
The photoinduced absorption changes depended strongly on the presence or absence of electron acceptors and donors in the system (Fig. 3). In the absence of oxygen, the photoinduced generation of Rh(IV) was not observed or the original Rh(IV)-concentration decreased upon irradiation. This indicated that shorter wavelength light does not work, because electrons excited from Rh(III) to the titania CB must be consumed by oxygen adsorbed on the surface. In contrast, the presence of AcH enhanced photoinduced regeneration of Rh(III); higher and lower concentrations of Rh(III) and Rh(IV), respectively, were observed for all wavelength ranges. This suggested that the efficiency of the photoinduced reduction of Rh(IV) may be improved by increasing the consumption of the VB positive holes by AcH. The Rh(IV)/Rh(III) ratio also decreased slowly in the dark in the presence of AcH, indicating that AcH reduces Rh(IV) slowly (ESI†).
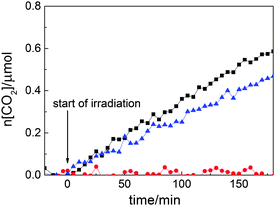
Thus, Rh–TiO2 photocatalysis contains at least two independent photoexcitation processes: excitations from Rh(III) to the titania CB by 440–550 nm light, and from the titania VB to Rh(IV) by irradiation with wavelengths from 590 to 730 nm. To confirm the synergy of these two photoreactions, the experiments shown in Fig. 4 were performed (ESI†), in which the full output (440–730 nm) of a xenon arc was divided into two wavelength ranges, 440–550 nm and 590–730 nm (provided by two light sources ensuring similar radiation intensities (ESI†)), which corresponded to photoabsorption by Rh(III) and Rh(IV), respectively. Negligible photocatalytic activity under the longer wavelength irradiation was attributed to the lower concentration of Rh(IV) excited, which could not be generated under these irradiation conditions, and the accumulation of electrons in Rh(III) without transfer to oxygen. However, the rate of CO2 liberation when irradiated in both wavelength ranges was higher than that under irradiation with shorter wavelength light. The synergetic effect was observed for 0.01% Rh–TiO2, whereas no synergetic effect over the full range of irradiation was observed for 0% Rh–TiO2. Analysis of the absorption spectra of 0.01% Rh–TiO2 recorded during the full-range irradiation in the presence of AcH showed negligible detectable change in the Rh(III)/Rh(IV) ratio. In contrast, shorter wavelength irradiation caused Rh(IV) to accumulate because of the slow reduction by AcH without photoinduced reduction, which might induce the slow deactivation of the sample under these conditions (Fig. 3, lower panel). A slightly higher increase of the reaction rate upon full-range irradiation observed at the beginning of the reaction might be explained by the recapturing of active Rh(III) through the reduction of Rh(IV) intrinsically present in the sample.
Based on these results and discussion, a schematic representation of the mechanism of the present photocatalytic reaction system with Rh–TiO2 is shown in Fig. 5. Higher energy photons (440–590 nm) excite electrons in Rh(III) into the titania CB to leave Rh(IV), whereas lower energy photons (590–730 nm) excite electrons in the titania VB to Rh(IV) to leave positive holes in the VB and to recover Rh(III). As a result, CB electrons and VB positive holes are generated by two types of visible-light photons, and a two-photon band-gap excitation proceeds using the Rh species as a built-in redox mediator. This mechanism is supported by theoretical calculations, which showed that Rh-doping of rutile titania should induce intermediate bands within the titania band gap.15–17 This photocatalyst has at least three advantages. First, the two different excitation processes occur in each titania particle because of the built-in redox mediator, which is different to Z-scheme photocatalysis using two kinds of photocatalyst particles.18–21 Second, the titania maintains a sufficient redox ability because of the band-gap excitation. Third, the two excitations require different ranges of light wavelengths that do not overlap. Because ordinary light sources, such as solar radiation, contain light with a wide range of wavelengths covering both excitation processes in the photocatalyst, the overall efficiency is not decreased even if two photons are required to produce the band-gap excitation.
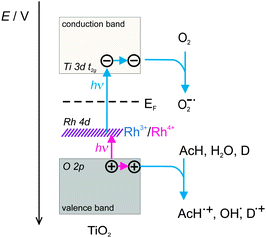 | ||
| Fig. 5 Schematic representation of the mechanism of the photocatalytic reaction with Rh–TiO2 (D – electron donor). | ||
At present, the overall efficiency is too low to be competitive with rutile titania under visible-light irradiation. This must be improved by increasing the concentration of active Rh species and by avoiding the formation of large aggregates of Rh oxide. This could be achieved by true doping of Rh(IV) ions in the titania lattice, which should be possible because the ionic radius of Rh(IV) is similar to that of Ti(IV) and the valency and coordination of Rh(IV) and Ti(IV) are the same. We are currently investigating this.
The present mechanism of the two-photon band-gap excitation of titania is, as far as we know, the sole possible solution for overcoming the main disadvantage of a titania photocatalyst that is not excited by visible light, without losing the advantages of the catalyst. The mechanism also provides a new insight into visible-light-induced photocatalysis by metal–oxide particles.
This work was supported by the Ministry of Science and Higher Education in Poland within Iuventus Plus grant (No. IP 2012030572).
References
- A. Fujishima, X. Zhang and D. A. Tryk, Surf. Sci. Rep., 2008, 63, 515–582 CrossRef CAS PubMed.
- R. Asahi, T. Morikawa, T. Ohwaki, K. Aoki and Y. Taga, Science, 2001, 293, 269–271 CrossRef CAS PubMed.
- T. Umebayashi, T. Yamaki, H. Itoh and K. Asai, Appl. Phys. Lett., 2002, 81, 454–456 CrossRef CAS PubMed.
- H. Irie, Y. Watanabe and K. Hashimoto, Chem. Lett., 2003, 32, 772–773 CrossRef CAS.
- Y. Nosaka, S. Takahashi, H. Sakamoto and A. Y. Nosaka, J. Phys. Chem. C, 2011, 115, 21283–21290 CAS.
- W. Choi, A. Termin and M. R. Hoffmann, J. Phys. Chem., 1994, 98, 13669–13679 CrossRef.
- R. Niishiro, R. Konta, H. Kato, W.-J. Chun, K. Asakura and A. Kudo, J. Phys. Chem. C, 2007, 111, 17420–17426 CAS.
- A. K. P. D. Savio, J. Fletcher and F. C. R. Hernandez, Ceram. Int., 2013, 39, 2753–2765 CrossRef CAS PubMed.
- Y. Matsumoto, T. Shimizu, A. Toyoda and E. Sato, J. Phys. Chem., 1982, 86, 3581 CrossRef CAS.
- R. Konta, T. Ishii, H. Kato and A. Kudo, J. Phys. Chem. B, 2004, 108, 8992–8995 CrossRef CAS.
- K. Iwashina and A. Kudo, J. Am. Chem. Soc., 2011, 133, 13272–13275 CrossRef CAS PubMed.
- K. Furuhashi, Q. Jia, A. Kudo and H. Onishi, J. Phys. Chem. C, 2013, 117, 19101–19106 CAS.
- H. W. Kang and S. B. Park, Int. J. Hydrogen Energy, 2013, 38, 823–831 CrossRef CAS PubMed.
- P. Shen, J. C. Lofaro Jr., W. R. Woerner, M. G. White, D. Su and A. Orlov, Chem. Eng. J., 2013, 223, 200–208 CrossRef CAS PubMed.
- F. E. Oropeza and R. G. Egdell, Chem. Phys. Lett., 2011, 515, 249–253 CrossRef CAS PubMed.
- K. K. Ghuman and C. V. Singh, J. Phys.: Condens. Matter, 2013, 25, 475501 CrossRef PubMed.
- K. Song, X. Han and G. Shao, J. Alloys Compd., 2013, 551, 118–124 CrossRef CAS PubMed.
- Y. Sasaki, H. Nemoto, K. Saito and A. Kudo, J. Phys. Chem. C, 2009, 113, 17536–17542 CAS.
- K. Sayama, K. Mukasa, R. Abe, Y. Abe and H. Arakawa, J. Photochem. Photobiol., A, 2002, 148, 71–77 CrossRef CAS.
- Y. Sasaki, A. Iwase, H. Kato and A. Kudo, J. Catal., 2008, 259, 133–137 CrossRef CAS PubMed.
- R. Abe, J. Photochem. Photobiol., C, 2010, 11, 179–209 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Preparation procedure and experimental details, XRD patterns of Rh–TiO2 samples, example of the time-course curve of photocatalytic test reaction, dependency of photocatalytic reaction rate on Rh-concentration determined for the materials calcined at 973 K, differential absorption spectra recorded for 0.01% Rh–TiO2 kept in a dark in the presence of AcH and irradiated. See DOI: 10.1039/c4cc07049f |
| This journal is © The Royal Society of Chemistry 2015 |