 Open Access Article
Open Access ArticleReversible capping/uncapping of phosphorous-centered Keggin-type polyoxoniobate clusters†
Jung-Ho
Son
*a and
William H.
Casey
*b
aDepartment of Chemistry, University of California, Davis One Shields Ave. Davis, CA 95616, USA. E-mail: junghoson@gmail.com
bDepartment of Chemistry, Department of Earth and Planetary Sciences, University of California, Davis, One Shields Ave. Davis, CA 95616, USA. E-mail: whcasey@ucdavis.edu; Fax: +1 530 752 8995
First published on 27th October 2014
Abstract
Caps in α-Keggin-type polyoxometalates [PM2Nb12O40]9− (M: Nb![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O or V
O or V![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) can be removed in basic condition to produce uncapped [PNb12O40]15−. Transmetalation or capping occurs from the reaction of [PNb14O42]9− or [PNb12O40]15− with either Sb2O3 or V2O5 to form [PSb2Nb12O40]9− or [PV2Nb12O42]9−, respectively.
O) can be removed in basic condition to produce uncapped [PNb12O40]15−. Transmetalation or capping occurs from the reaction of [PNb14O42]9− or [PNb12O40]15− with either Sb2O3 or V2O5 to form [PSb2Nb12O40]9− or [PV2Nb12O42]9−, respectively.
One interesting mode of heterometal addition to the Keggin-type polyoxometalate clusters is capping, whereby a capping metal reduces the overall charge of the cluster. Several heterometals, such as VIV,V,1 NiII,2 CuII,3 ZnII,4 SbIII,5 and LaIII,6 are known as caps; among these VIV,V caps are the most common. The number of capping site can vary from 1 to 6. The capped Mo-, V- or W-based Keggin-type clusters have proven to be useful for spintronics7 and as supramolecular materials for catalytic applications.4,8 For the polyoxoniobates, vanadyl-capped Keggin-type polyoxoniobate ions have been synthesized recently, including [PV2Nb12O42]9− ion (PV2Nb12).9 Here we expand the library of capped Keggin polyoxoniobates as TMA (tetramethylammonium) salts; SbIII- or NbV
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O-bicapped α-Keggin polyoxoniobates TMA9[PSb2Nb12O40]·28H2O (PSb2Nb12) and TMA9[PNb14O42]·26H2O (PNb14). We also isolated novel TMA10H5[PNb12O40]·30.5H2O (PNb12) via an uncapping reaction starting from PNb14 in highly basic condition. The synthesis of discrete PNb14 clusters is important because NbV-bicapped Keggin niobates have only been characterized as chain structures formed by Nb–(μ2-O)2–Nb bridges.10
O-bicapped α-Keggin polyoxoniobates TMA9[PSb2Nb12O40]·28H2O (PSb2Nb12) and TMA9[PNb14O42]·26H2O (PNb14). We also isolated novel TMA10H5[PNb12O40]·30.5H2O (PNb12) via an uncapping reaction starting from PNb14 in highly basic condition. The synthesis of discrete PNb14 clusters is important because NbV-bicapped Keggin niobates have only been characterized as chain structures formed by Nb–(μ2-O)2–Nb bridges.10
Here we show that capping/uncapping reaction can be reversed for vanadyl capping group. Although many kinds of capped-Keggin clusters are known, reversibility is not commonly shown, nor well understood. We believe that reaction studies at the capping site in the Keggin ion can be particularly useful for polymerization studies that exploit reactions at the caps. We note that a controlled capping reaction of [PMo12O40]3− by using electrochemical reduction to produce CoII-, VIV-, and SbIII-capped Keggin ion has been reported previously.11
The PSb2Nb12 cluster (Fig. 1) was synthesized by hydrothermal reaction of the mixture of stoichiometric amounts of hydrous niobium oxide, Sb2O3, TMAOH and phosphoric acid. In the crystal structure, two distinct PSb2Nb12 clusters are present in the crystallographic lattice; one of them has a pseudo-Keggin structure, which features central PO8 with half-occupied oxygen atoms due to rotational disorder. Nearly nine TMA countercations are found per cluster, so the cluster formula is [PSbIII2Nb12O40]9−. Bond-valence-sum (BVS) values for three antimony sites are 3.14, 3.07 and 3.26, which agree with the oxidation state of SbIII. Electrospray-ionization mass spectrometry (ESI-MS) of the compound provided spectra consistent with the stoichiometry, and the peaks are finely split due to the natural isotopes of antimony (Fig. S1, ESI†).
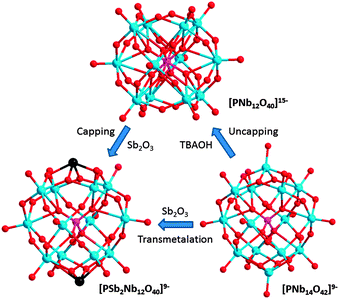 | ||
| Fig. 1 Ball-and-stick models (pink: P, red: O, light blue: Nb, black: Sb) of PNb12 (top), PSb2Nb12 (bottom left) and PNb14 (bottom right) clusters. | ||
A different Keggin-type cluster formed when the hydrothermal reaction was carried out without Sb2O3. The product was generally waxy and we crystallized it in a hot concentrated ethanol solution. The crystal structure shows the cluster of [PNb14O42]9−, with two bicapping trans NbV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (Fig. 1). Seven TMA ions were found in the crystal structure, but elemental analysis and TGA data (Fig. S2, ESI†) of the compound better agrees with nine TMA, as was the case in PSb2Nb12 and PV2Nb12. We thus conclude that two TMA are disordered in the solvent region and thus could not be found during the structure refinement. In the ESI-MS spectra, the products always showed small peaks with lower m/z number and we tentatively assign this impurity as the mono-capped [PNb13O41]12− (PNb13) (Fig. S1, ESI†). A GeIV-centered [GeNb13O41]13− as Cs+ or Rb+ salt was structurally characterized recently, and this also supports our assignment of the small impurity peaks as the PNb13.12
O (Fig. 1). Seven TMA ions were found in the crystal structure, but elemental analysis and TGA data (Fig. S2, ESI†) of the compound better agrees with nine TMA, as was the case in PSb2Nb12 and PV2Nb12. We thus conclude that two TMA are disordered in the solvent region and thus could not be found during the structure refinement. In the ESI-MS spectra, the products always showed small peaks with lower m/z number and we tentatively assign this impurity as the mono-capped [PNb13O41]12− (PNb13) (Fig. S1, ESI†). A GeIV-centered [GeNb13O41]13− as Cs+ or Rb+ salt was structurally characterized recently, and this also supports our assignment of the small impurity peaks as the PNb13.12
In the structure of PNb14, capping NbV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O are slightly tilted from the pseudo-C4 rotational axis of the Keggin ion (angles of P1–Nb13–O41 = 173.04° and P1–Nb14–O42 = 170.24°) (Fig. 1 and Fig. S3, ESI†). Thermal ellipsoids of the capping NbV
O are slightly tilted from the pseudo-C4 rotational axis of the Keggin ion (angles of P1–Nb13–O41 = 173.04° and P1–Nb14–O42 = 170.24°) (Fig. 1 and Fig. S3, ESI†). Thermal ellipsoids of the capping NbV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O oxygen atoms are horizontally elongated compared to other terminal oxygen atoms, and the NbV
O oxygen atoms are horizontally elongated compared to other terminal oxygen atoms, and the NbV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond lengths (1.726(13) and 1.735(12) Å) at the capping sites are slightly shorter than other NbV
O bond lengths (1.726(13) and 1.735(12) Å) at the capping sites are slightly shorter than other NbV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds (1.741(9) to 1.774(9) Å). These might be due to the rare pentacoordinate NbV environment in the capping site,13 and explain the reactivity of NbV
O bonds (1.741(9) to 1.774(9) Å). These might be due to the rare pentacoordinate NbV environment in the capping site,13 and explain the reactivity of NbV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O capping site as described below.
O capping site as described below.
We found that bicapping NbV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O units in PNb14 can be uncapped under strongly basic conditions. When PNb14 was mixed with TBAOH solution in an open vial and kept at 85 °C in a dry oven overnight, crystalline materials of PNb12 formed at the bottom of the vial. ESI-MS peaks of the newly formed PNb12 appear in lower m/z region relative to spectra for the PNb14 and match well with the composition of PNb12 identified in the crystal structure (Fig. S1, ESI†). We see an additional peak at m/z = 426.6 and tentatively assign it to a lacunary H14[PNb11O39](H2O)4− ion, which could have formed by fragmentation in ESI-MS.
O units in PNb14 can be uncapped under strongly basic conditions. When PNb14 was mixed with TBAOH solution in an open vial and kept at 85 °C in a dry oven overnight, crystalline materials of PNb12 formed at the bottom of the vial. ESI-MS peaks of the newly formed PNb12 appear in lower m/z region relative to spectra for the PNb14 and match well with the composition of PNb12 identified in the crystal structure (Fig. S1, ESI†). We see an additional peak at m/z = 426.6 and tentatively assign it to a lacunary H14[PNb11O39](H2O)4− ion, which could have formed by fragmentation in ESI-MS.
The PNb12 cluster in the crystal structure exhibits a pseudo-Keggin structure, similar to one of the clusters in PSb2Nb12 structure (Fig. 1). The O⋯O distances between the square-like window for capping (2.65–2.70 Å) in PNb12 are less contracted than those in the SbIII-capped window of same pseudo-Keggin unit in PSb2Nb12 (2.50–2.51 Å), due to the absence of capping metal. Generally, the O⋯O distances in the capping site are in the order of PNb12 > PNb14 > PSb2Nb12 > PV2Nb12 (Fig. S4 and S5, ESI†). Although PNb12 should possess a −15 charge, only 10 TMA ions are found in the crystal structure, and this number agrees with elemental analyses and TGA data (Fig. S2, ESI†). We propose that five protons are disordered on the PNb12 cluster surface, but we are unable to assign the protonation sites by BVS values (1.57 to 1.86) of the surface μ2-oxygens. We note that uncapped PNb12 Keggin structure has not been reported as a soluble form so far.
The 31P MAS-NMR data of the synthesized Keggin compounds are shown in Fig. 2. The chemical shifts of each Keggin structure are slightly different, with 5.2, 4.0 and 2.4 ppm for PSb2Nb12, PNb14 and PNb12, respectively. The downfield 31P peak shift of PSb2Nb12 and PNb14 compared to PNb12 can be attributed to the existence of capping atoms (SbIII or NbV), and more downfield shift in PSb2Nb12 is attributed to higher electronegativity of antimony compared to niobium. The spectra of PNb14 features additional small peak at 1.04 ppm. We propose that the small peak arises from PNb13, as indicated by ESI-MS (Fig. S1, ESI†). The 31P-NMR peak of PNb12 is broader than the peaks of bicapped Keggin compounds, due to less symmetric P–O bonds at the center of the structure. The P–O bonds in PNb12 range 1.500(10)–1.628(11) Å, while the P–O bonds in PSb2Nb12 and PNb14 have more regular P–O bonds (1.529(7)–1.594(7) Å and 1.544(9)–1.559(8) Å, respectively).
 | ||
| Fig. 2 31P MAS NMR (left) and 31P solution NMR (right) spectra. The apparent peaks at 2.8 ppm (right) are instrumental artifacts. | ||
Stability of the clusters in solution was checked by using 31P NMR (Fig. 2). When the compounds were dissolved in D2O, PSb2Nb12, PNb14 and PNb12 showed peaks at 6.4, 5.4 and 5.2 ppm, respectively, and the peak from PNb12 was broad, similarly to MAS NMR. The PNb14 showed large amount of unassignable broad peaks upfield, suggesting polymerization of PNb14 in water. In methanol, PNb14 shows two peaks at 4.8 and 3.9 ppm with integral ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3, which might correspond to PNb14 and PNb13. The sharp peaks in methanol suggest that the series of broad peaks of PNb14 in D2O are due to polymerization of the molecules in water. FT-IR spectra (Fig. S6, ESI†) of the three compounds are all similar to that of previously reported PV2Nb12, featuring P–O band around 1025 cm−1, Nb
0.3, which might correspond to PNb14 and PNb13. The sharp peaks in methanol suggest that the series of broad peaks of PNb14 in D2O are due to polymerization of the molecules in water. FT-IR spectra (Fig. S6, ESI†) of the three compounds are all similar to that of previously reported PV2Nb12, featuring P–O band around 1025 cm−1, Nb![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O band around 880 cm−1 and some Nb–O–Nb bands between 850–600 cm−1.14 We note that the FT-IR spectrum of PNb12 generally shows broader bands than other compounds, possibly due to its less compact structure from the absence of capping ions.
O band around 880 cm−1 and some Nb–O–Nb bands between 850–600 cm−1.14 We note that the FT-IR spectrum of PNb12 generally shows broader bands than other compounds, possibly due to its less compact structure from the absence of capping ions.
The stabilities of these clusters were monitored by ESI-MS as a function of pH (Fig. S7 to S9, ESI†). PSb2Nb12 was seen to be stable between 4 < pH < 12, similar to the stability range of PV2Nb12. The PNb12 was stable in the higher pH region (6 < pH < 12), which is consistent with its formation condition at high pH and its high molecular charge. A solution of PNb12 formed precipitate when pH was reduced to below pH = 6, consistent with charge neutralization. In contrast, The PNb14 cluster exhibited a narrower pH stability range (8 < pH < 12), and the cluster was unstable when titrated with acid.
Both PNb14 and PNb12 can directly react with Sb2O3 or V2O5 to form PSb2Nb12 or PV2Nb12 by simple solution reaction at high yields. By using ESI-MS, we monitored the reaction in a capped vial at 100 °C and 70 °C for antimony and vanadium capping, respectively (Fig. S10 and S11, ESI†). The complete formation of PSb2Nb12 was slower (∼2 h) than PV2Nb12 (<1 h). When starting from PNb14, the formation of hetero-capped Keggin ions such as [PSbNb13O41]9− or [PVNb13O42]9− as intermediates was detected by using ESI-MS. The capping reaction of PNb12 was also monitored similarly, and the reaction proceeds with intermediates such as mono-capped [PVNb12O41]12− or [PSbNb12O40]12− (Fig. 3 and Fig. S11, ESI†). Thus the reaction apparently occurs via stepwise substitution from PNb14, or addition of capping sites to PNb12, respectively. These reactions can be completed even at room temperature after stirring the mixture for a few days. Because the conversion of PNb14 to PSb2Nb12 or PV2Nb12 apparently involves direct substitution of the capping unit from NbV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O to SbIII or VV
O to SbIII or VV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, this reaction can be regarded as transmetalation. Transmetalation is a well-known synthetic strategy in organometallic chemistry, but rare in polyoxometalates to our knowledge.15 Similarly to the uncapping reaction of PNb14 to form PNb12, PV2Nb12 can also be uncapped to form PNb12 in a same condition, thus uncapping/capping reaction of VV
O, this reaction can be regarded as transmetalation. Transmetalation is a well-known synthetic strategy in organometallic chemistry, but rare in polyoxometalates to our knowledge.15 Similarly to the uncapping reaction of PNb14 to form PNb12, PV2Nb12 can also be uncapped to form PNb12 in a same condition, thus uncapping/capping reaction of VV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O is reversible (see experimental section in ESI†). The PSb2Nb12 could not be uncapped even with larger amount of base added during attempted reaction.
O is reversible (see experimental section in ESI†). The PSb2Nb12 could not be uncapped even with larger amount of base added during attempted reaction.
We demonstrate that P-centered Keggin polyoxoniobate can be capped or uncapped at certain reaction conditions, and the reaction depends on the stability of the capped niobate. Such a capping or transmetalation reaction, starting from PNb12 or PNb14, is an attractive route for selective capping by various types of transition metals and allows unprecedented control. This control over capping/uncapping can be particularly useful for polymerizing Keggin ions by exploiting the heterogeneity of opposed apical bicaps. The newly isolated and discrete PNb14 ion can be used as a precursor for rational synthesis of oligomeric chains of Keggin ions in materials science.
This work was supported by an NSF CCI grant through the Center for Sustainable Materials Chemistry, number CHE-1102637. Additional support to JHS was via NSF-CHE-1310368 to WHC. The authors thank Dr Ping Yu, Corey Pilgrim and Gerry Ochoa for help collecting the NMR spectra. We also thank Dana Reusser and Prof. Alex Navrotsky for TGA data.
Notes and references
- (a) Q. Chen and C. L. Hill, Inorg. Chem., 1996, 35, 2403–2405 CrossRef CAS; (b) M. Yuan, Y. Li, E. Wang, Y. Lu, C. Hu, N. Hu and H. Jia, J. Chem. Soc., Dalton Trans., 2002, 2916–2920 RSC; (c) M. Yuan, Y. Li, E. Wang, C. Tian, L. Wang, C. Hu, N. Hu and H. Jia, Inorg. Chem., 2003, 42, 3670–3676 CrossRef CAS PubMed; (d) J. Sha, J. Peng, H. Liu, J. Chen, A. Tian and P. Zhang, Inorg. Chem., 2007, 46, 11183–11189 CrossRef CAS PubMed; (e) J. Sha, J. Peng, S. Zhou, M. Zhu, L. Han and D. Chen, J. Cluster Sci., 2008, 19, 499–509 CrossRef CAS; (f) J. Fu, H. Sun, Y. Xu, C. Wang, D. Zhu, Q. Sun and H. Liu, CrystEngComm, 2012, 14, 5148–5150 RSC.
- (a) J.-W. Cui, X.-B. Cui, H.-H. Yu, J.-Q. Xu, Z.-H. Yi and W.-J. Duan, Inorg. Chim. Acta, 2008, 361, 2641–2647 CrossRef CAS PubMed; (b) W. Wang, L. Xu, G. Gao, L. Liu and X. Liu, CrystEngComm, 2009, 11, 2488–2493 RSC.
- Y. Bai, Y. Li, E. Wang, X. Wang, Y. Lu and L. Xu, J. Mol. Struct., 2005, 752, 54–59 CrossRef CAS PubMed.
- B. Nohra, H. El Moll, L. M. Rodriguez-Albero, P. Mialane, J. Marrot, C. Mellot-Draznieks, M. O'Keeffe, R. Ngo Biboum, J. Lemaire, B. Keita, L. Nadjo and A. Dolbecq, J. Am. Chem. Soc., 2011, 133, 13363–13374 CrossRef CAS PubMed.
- (a) S.-Y. Shi, H.-H. Teng, L.-M. Chang, Y. Wang, L.-N. Xiao, X.-B. Cui and J.-Q. Xu, Inorg. Chim. Acta, 2013, 399, 172–176 CrossRef CAS PubMed; (b) J. Huang, Z. Han, H. Zhang, H. Yu and X. Zhai, J. Solid State Chem., 2012, 194, 65–70 CrossRef CAS PubMed; (c) Y.-K. Lu, J.-N. Xu, X.-B. Cui, J. Jin, S.-Y. Shi and J.-Q. Xu, Inorg. Chem. Commun., 2010, 13, 46–49 CrossRef CAS PubMed.
- (a) A. Dolbecq, P. Mialane, L. Lisnard, J. Marrot and F. Sécheresse, Chem. – Eur. J., 2003, 9, 2914–2920 CrossRef CAS PubMed; (b) P. Mialane, A. Dolbecq, L. Lisnard, A. Mallard, J. Marrot and F. Sécheresse, Angew. Chem., Int. Ed., 2002, 41, 2398–2401 CrossRef CAS.
- (a) J. Lehmann, A. Gaita-Ariño, E. Coronado and D. Loss, J. Mater. Chem., 2009, 19, 1672 RSC; (b) J. Lehmann, A. Gaita-Ariño, E. Coronado and D. Loss, Nat. Nanotechnol., 2007, 2, 312 CrossRef CAS PubMed.
- (a) A. Müller, M. Koop, P. Schiffels and H. Bögge, Chem. Commun., 1997, 1715–1716 RSC; (b) X. Wang, L. Liu, G. Zhang and A. J. Jacobson, Chem. Commun., 2001, 2472–2473 RSC; (c) Z. Zhang, Q. Lin, D. Kurunthu, T. Wu, F. Zuo, S.-T. Zheng, C. J. Bardeen, X. Bu and P. Feng, J. Am. Chem. Soc., 2011, 133, 6934–6937 CrossRef CAS PubMed.
- (a) G. Guo, Y. Xu, J. Cao and C. Hu, Chem. Commun., 2011, 47, 9411–9413 RSC; (b) J.-H. Son, C. A. Ohlin, E. C. Larson, P. Yu and W. H. Casey, Eur. J. Inorg. Chem., 2013, 1748–1753 CrossRef CAS; (c) J.-H. Son, C. A. Ohlin, R. L. Johnson, P. Yu and W. H. Casey, Chem. – Eur. J., 2013, 19, 5191–5197 CrossRef CAS PubMed; (d) J.-Q. Shen, Y. Zhang, Z.-M. Zhang, Y.-G. Li, Y.-Q. Gao and E.-B. Wang, Chem. Commun., 2014, 50, 6017–6019 RSC.
- (a) M. Nyman, J. P. Larentzos, E. J. Maginn, M. E. Welk, D. Ingersoll, H. Park, J. B. Parise, I. Bull and F. Bonhomme, Inorg. Chem., 2007, 46, 2067–2079 CrossRef CAS PubMed; (b) F. Bonhomme, J. P. Larentzos, T. M. Alam, E. J. Maginn and M. Nyman, Inorg. Chem., 2005, 44, 1774–1785 CrossRef CAS PubMed; (c) M. Nyman, F. Bonhomme, T. M. Alam, M. A. Rodriguez, B. R. Cherry, J. L. Krumhansl, T. M. Nenoff and A. M. Sattler, Science, 2002, 297, 996–998 CrossRef CAS PubMed.
- R. Bakri, A. Booth, G. Harle, P. S. Middleton, C. Wills, W. Clegg, R. W. Harrington and R. J. Errington, Chem. Commun., 2012, 48, 2779–2781 RSC.
- Y. Hou, L. N. Zakharov and M. Nyman, J. Am. Chem. Soc., 2013, 135, 16651–16657 CrossRef CAS PubMed.
- (a) G. Meyer, R. Hoppe and M. Jansen, Naturwissenschaften, 1976, 63, 386 CrossRef CAS; (b) M. H. Chisholm, A. H. Cowley and M. Lattman, J. Am. Chem. Soc., 1980, 102, 46–50 CrossRef CAS; (c) G. S. McGrady, A. Haaland, H. P. Verne, H. V. Volden, A. J. Downs, D. Shorokhov, G. Eickerling and W. Scherer, Chem. – Eur. J., 2005, 11, 4921–4934 CrossRef CAS PubMed.
- C. Rocchiccioli-Deltcheff, M. Fournier, R. Franck and R. Thouvenot, Inorg. Chem., 1983, 22, 207–216 CrossRef CAS.
- M. E. Carnes, M. S. Collins and D. W. Johnson, Chem. Soc. Rev., 2014, 43, 1825–1834 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, crystallographic table, TGA, FT-IR, ESI-MS spectra and pH dependent ESI-MS spectra of the compounds, ESI-MS while monitoring the reactions. CCDC 1014963–1014965. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4cc05689b |
| This journal is © The Royal Society of Chemistry 2015 |

