 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
MNAzyme-catalyzed nucleic acid detection enhanced by a cationic copolymer†
Jueyuan
Gao
a,
Naohiko
Shimada
b and
Atsushi
Maruyama
*b
aGraduate School of Engineering, Kyushu University, 744 Motooka, Nishi, Fukuoka 819-0395, Japan
bDepartment of Biomolecular Engineering, Graduate School of Bioscience and Biotechnology, Tokyo Institute of Technology, 4259-B57 Nagatsuta, Midori, Yokohama, 226-8501, Japan. E-mail: amaruyama@bio. titech.ac.jp
First published on 11th March 2015
Abstract
Multi-component nucleotide acid enzymes (MNAzymes) derived from RNase-mimic DNAzymes have potential as simple and accurate DNA detectors. To enhance the MNAzyme activity under multiple-turnover conditions, a cationic comb-type copolymer, PLL-g-Dex, that facilitates hybridization and strand exchange reactions of DNA was utilized. The copolymer increased the MNAzyme reaction rate by 200 times, allowing target DNA detection at picomolar concentrations at physiological temperature.
Introduction
The emergence of life-threatening infections such as severe acute respiratory syndrome (SARS) and viral haemorrhagic fevers caused by the Ebola and Marburg viruses highlights the urgent need for efficient infection control practices. High-speed, accurate, and simple methods to detect pathogens that can be used in field hospitals are required. Such methods are also important for establishing point-of-care diagnostic capabilities. Isothermal amplification methods,1,2 such as rolling circle amplification (RCA),3,4 loop-mediated isothermal amplification (LAMP),5 and helicase-dependent amplification (HAD),6 to detect pathogen genetic material or disease markers have advantages over current PCR-based methods because of their simple protocols and because they could be conducted at ambient temperature. However, these isothermal amplification methods still have complicated setups and require multiple enzymes and/or primers.Multi-component nucleic acid enzyme (MNAzyme)-based assays7,8 are another type of isothermal, signal amplifying DNA-detection method. MNAzymes9,10 are composed of short oligonucleotides that function as partial enzymes or partzymes. Each partzyme includes a region of a catalytic core, a substrate-binding arm, and a sensor arm. The sensor arms recognize and bind to a target nucleic acid. When the partzymes are assembled in the presence of a target nucleic acid, they form an active MNAzyme assembly that catalytically produces signals. The signal results from substrate cleavage, generally upon release of a fluorophore from proximity to a quencher. As multiple signals can be generated from a single target molecule, isothermal signal amplification is possible. The MNAzyme integrated with various detection methods has been widely investigated.11–16
We previously reported that a cationic comb-type copolymer consisting of a polycationic backbone and hydrophilic graft chains of dextran (Fig. 1A) promoted hybridization of a pair of complementary DNAs.17–19 The copolymer also facilitates the strand exchange reaction between a double-stranded DNA and homologous single-stranded DNA.20–22 Recently, we showed that the copolymer considerably enhanced ribonuclease activity of the previously described 10–23 DNAzymes.23 The copolymer facilitated turnover of the DNAzyme and stabilized the DNAzyme over a wide range of temperature.23
Here, we show that the cationic copolymer also enhances activity of an MNAzyme derived from the 10–23 DNAzyme and increased MNAzyme sensitivity. Furthermore, the copolymer enabled us to shorten the substrate-binding arms of the MNAzyme, decreasing the optimum temperature of the MNA assay from 50 °C to physiological temperature.
Experiment
Materials
Poly(L-lysine hydrobromide) (PLL·HBr, MW = 7.5 × 103) was obtained from Sigma-Aldrich Co. LLC (St Louis, MO, USA), and dextran (Dex, MW = 8 × 103–1.2 × 104) was obtained from Funakoshi Co. (Tokyo, Japan). Sodium hydroxide, sodium chloride, magnesium sulfate, and manganese(II) chloride tetrahydrate were purchased from Wako Pure Chemical Industries (Osaka, Japan). 2-[4-(2-Hydroxyethyl)piperazin-1-yl]ethanesulfonic acid (HEPES) and ethylene diamine tetraacetic acid tetrasodium salt (EDTA·4Na) were purchased from Nacalai Tesque, Inc. (Kyoto, Japan). HPLC-grade oligonucleotides with sequences shown in Fig. 1 were purchased from Fasmac Co., Ltd (Atsugi, Japan) and used without further purification. A cationic comb-type copolymer poly(L-lysine)-graft-dextran (PLL-g-Dex) was prepared according to the previously published procedure.18 Briefly, PLL-g-Dex was prepared by a reductive amination reaction of dextran with PLL. The resulting copolymer was isolated through an ion exchange column, dialyzed, and lyophilized. The product was characterized by 1H NMR and GPC. PLL-g-Dex of 90 wt% dextran (11.5 mol% of lysine units of PLL were modified with dextran) was used in this study.Methods
For FRET analyses, substrate (final concentration: 200 nM) and MNAzyme parts (final concentrations: 200 nM, 20 nM, 6.7 nM, or 2 nM) were dissolved in a reaction buffer, 50 mM HEPES (pH 7.3), 150 mM NaCl, 25 mM Mg2+ or 5 mM Mn2+. The mixture of substrate and MNAzyme parts was pre-incubated with or without PLL-g-Dex at reaction temperature for 15 min in a quartz cell. The ratio of positively charged amino groups of the copolymer to negatively charged phosphate groups of DNA (N/P ratio) was 2. After pre-incubation, 20 μL target solution (final concentrations: 2 pM–50 nM) was injected into the cell (final volume of reaction solution was 1800 μL) to initiate the reaction. The fluorescence intensity was measured using a FP-6500 spectrofluorometer (Jasco, Tokyo, Japan) at an excitation wavelength, λex, of 494 nm and an emission wavelength, λem, of 520 nm. The percent cleavage (P%) as a function of time was obtained from the equation P% = (It − I0)/(I∞ − I0), where It is the fluorescence intensity at any reaction time t, I∞ is the fluorescence intensity of the synthesized product, and I0 is the initial fluorescence intensity (background). The values of kobs were obtained by fitting the reaction curve with the equation It = I0 + (I∞ − I0) (1 − e−kobst).Results and discussion
Firstly, we examined MNAzyme A, which has longer substrate-binding arms than MNAzyme B (Fig. 1B), activity. In initial experiments, the substrate and partzyme concentrations were 200 nM and the target was present at 2 nM. Buffer contained 25 mM Mg2+. Considerably higher cleavage activity was observed in the presence than in the absence of the PLL-g-Dex (Fig. 2), suggesting that the copolymer promoted turnover of the target. We then decreased the MNAzyme concentration while holding initial substrate and target concentrations constant. At lower MNAzyme concentrations we observed a significant decrease in MNAzyme activity in the absence of the copolymer, but only a slight decrease in the activity was observed in the presence of a copolymer even at 2 nM MNAzyme concentration (Fig. 2). This result indicated that the copolymer promoted target-induced assembly of an active MNAzyme complex at low MNAzyme concentrations. At 2 nM of each of the partzymes, the copolymer increased the cleavage rate by 50 fold relative to cleavage in the absence of copolymer.To estimate the target sensitivity of the MNAzyme, the target concentration dependence of the MNAzyme reaction was investigated. Fig. 3A shows target concentration dependence observed at substrate and MNAzyme concentrations of 200 nM in the absence of PLL-g-Dex. Under these conditions, the sensitivity was 2 orders of magnitude lower than that described previously,8 presumably owing to difference in length of the sensor arms that significantly influenced the stability of MNAzyme assembly. The sensor arms of MNAzyme in this study were 14 and 15 nucleotides (nt) whereas those in the previous study were 20 and 21 nt. In the presence of PLL-g-Dex, the sensitivity was enhanced approximately 5 times at an MNAzyme concentration of 2 nM (Fig. 3B), indicating that the copolymer significantly improved the MNAzyme reactivity under multiple-turnover conditions. This suggests that the use of the copolymer could enable use of the MNAzyme assay for detection of short nucleotide targets such as miRNA and siRNA.
In order to improve the MNAzyme sensitivity further, we investigated the effect of the metal ion cofactors. MNAzyme activity did not significantly change in 25 mM Mg2+ and 5 mM Mn2+ in the absence of the copolymer (Fig. S1†). Considerably higher MNAzyme activity was observed in 5 mM Mn2+ than in 25 mM Mg2+ when the copolymer was present (Fig. 3B and D). This is owing to the difference in the rate determining step of the MNAzyme reaction. While the rate determining step in the absence of the copolymer was the turnover process it was the chemical cleavage step in the presence of the copolymer.23 The target concentration dependences in the presence of 5 mM Mn2+ were determined in the presence and absence of PLL-g-Dex. The MNAzyme can detect the target at the nanomolar level without the copolymer (Fig. 3C), and it was at the picomolar level in the presence of PLL-g-Dex (Fig. 3D). As the reactions were initiated by the addition of the target to a mixture of MNAzymes and substrate, there was an initial lag during which the MNAzyme assembled. This lag was obvious in the absence of PLL-g-Dex (Fig. 3C) but was not observed in the presence (Fig. 3D), indicative of significant acceleration of MNAzyme assembly due to PLL-g-Dex. Fig. 3E summarizes the target concentration dependences of kobs. Compared with the sensitivity without the copolymer at 200 nM MNAzyme, that with the copolymer was improved more than two orders of magnitude. The sensitivity was in the pM range even at a 2 nM MNAzyme concentration when the copolymer was added to the reaction at an N/P of 2.
We previously reported that PLL-g-Dex enlarged the temperature window in which a DNAzyme was active.23 The requirement that temperature be cycled limits utility of the PCR-based assay in non-laboratory settings. If MNAzyme reactions are active over a wide range of temperature, these systems could be adapted to formats such as chromato-, nanoparticle- and microfluidics-based detection. We analyzed the temperature dependence of the multiple-turnover MNAzyme reaction at 200 nM substrate, 6.7 nM MNAzyme, and 2 nM target in a buffer containing Mn2+ in the absence and presence of the copolymer. As shown in Fig. 4, the optimum temperature of the MNAzyme reactions was increased by the copolymer. In the presence of the copolymer, kobs values were higher than 10−2 min−1 over the temperature range from 37 °C to 63 °C, whereas kobs was higher than 10−3 min−1 only between 37 °C and 55 °C without the copolymer. At 37 °C in the presence of the copolymer MNAzyme activity was higher than that obtained at the optimum temperature of 50 °C without the copolymer. At 50 °C the copolymer facilitated the reaction by approximately 100 fold. To further decrease the working temperature range of the MNAzyme we constructed MNAzyme B in which the substrate-binding arms were each truncated by 3 nt relative to the arms in MNAzyme A (Fig. 1B). As shown in Fig. 4, the optimal temperature for the reaction was lowered about 12 °C by the truncation. The truncation resulted in an extreme decrease in the MNAzyme activity in the absence of the copolymer. In the presence of PLL-g-Dex, however, the MNAzyme was active with kobs > 10−1 even at 37 °C.
The reactivity of MNAzymes was compared with that of their parent DNAzymes by analysis of the DNAzyme reactions at 200 nM substrate and 6.7 nM DNAzyme (Fig. S2†). The copolymer promoted multiple-turnover of the DNAzyme with substrate-binding arms comparable to those of MNAzyme A by approximately 10 fold, as reported previously.23 Interestingly, the effect of the copolymer was more pronounced with the DNAzyme with shorter substrate-binding arms. The 3 nt truncation considerably decreased stability of the DNAzyme/substrate (ES) complex as estimated by prediction of the melting temperature (Table S1†). The fact that the copolymer enhanced the efficiency of the less stable complex is interesting to note. The truncation of the substrate-binding arms should increase the dissociation rate of the DNAzyme/product complex, whereas PLL-g-Dex is expected to facilitate association of the substrate with the DNAzyme. These effects likely cooperated to facilitate the multiple-turnover reactions of the DNAzymes and MNAzymes with the short substrate-binding arms.
Finally, the target dependence of the MNAzyme B was evaluated at 37 °C with or without PLL-g-Dex under multiple-turnover conditions. As shown in Fig. 5, it took 60 min to cleave approximately 5% of the substrate in the absence of PLL-g-Dex, but it took less than a minute in the presence of PLL-g-Dex. The target was detected at picomolar concentrations in the presence of the copolymer at 37 °C. The limits of detection (LoD) of MNAzyme with and without PLL-g-Dex under these physiological conditions were determined (Table S2, Fig. S3†). The copolymer improved the LoD of MNAzyme about 100 times relative to the LoD under the same conditions but without PLL-g-Dex.
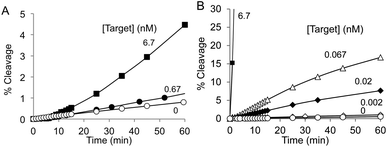 | ||
| Fig. 5 Target concentration dependence of MNAzyme B reactions in (A) the absence and (B) the presence (N/P = 2) of PLL-g-Dex at 37 °C in 5 mM MnCl2 at 200 nM substrate and 6.7 nM MNAzyme B. | ||
Conclusions
MNAzyme activity was significantly reduced under multiple-turnover conditions. In the presence of the cationic copolymer PLL-g-Dex, activity was significantly enhanced. The copolymer likely facilitated target-induced assembly of an active MNAzyme complex. Notably, with the copolymer and the short-armed MNAzyme DNA was detected at picomolar concentrations at physiological temperature. Although further improvement in the sensitivity will be required to enable utility in point-of-care diagnostic devices, use of the cationic copolymer will facilitate construction of simple and accurate assays using MNAzymes.Acknowledgements
Parts of this work were supported by a Grant-in-Aid for Scientific Research on Innovative Areas “Molecular Robotics” (no. 24104003), “Nanomedicine Molecular Science” (no. 2306) and the Cooperative Research Program of “Network Joint Research Center for Materials and Devices” from the Ministry of Education, Culture, Sports, Science and Technology, by Center of Innovation (COI) Program, Japan Science and Technology Agency (JST), and by KAKENHI (no. 23240074, 25350552) from the Japan Society for the Promotion of Science.Notes and references
- C. C. Chang, C. C. Chen, S. C. Wei, H. H. Lu, Y. H. Liang and C. W. Lin, Diagnostic devices for isothermal nucleic acid amplification, Sensor, 2012, 12, 8319–8337 CrossRef CAS PubMed.
- L. M. Zanoli and G. Spoto, Isothermal amplification methods for the detection of nucleic acids in microfluidic devices, Biosensor, 2013, 3, 18–43 CrossRef CAS PubMed.
- P. M. Lizardi, X. Huang, Z. Zhu, P. Bray-Ward, D. C. Thomas and D. C. Ward, Mutation detection and single-molecule counting using isothermal rolling-circle amplification, Nat. Genet., 1998, 19, 225–232 CrossRef CAS PubMed.
- J. Baner, M. Nilsson, M. Mendel-Hartvig and U. Landergren, Signal amplification of padlock probes by rolling circle replication, Nucleic Acids Res., 1998, 26, 5073–5078 CrossRef CAS PubMed.
- T. Notomi, H. Okayama, H. Masubuchi, T. Yonekawa, K. Watanabe, N. Amino and T. Hase, Loop-mediated isothermal amplification of DNA, Nucleic Acids Res., 2000, 28, e63 CrossRef CAS PubMed.
- M. Vincent, Y. Xu and H. Kong, Helicase-dependent isothermal DNA amplification, EMBO Rep., 2004, 5, 795–800 CrossRef CAS PubMed.
- Y. V. Gerasimova and D. M. Kolpashchikov, Nucleic acid detection using MNAzymes, Chem. Biol., 2010, 17, 104–106 CrossRef CAS PubMed.
- E. Mokany, S. M. Bone, P. E. Young, T. B. Doan and A. V. Todd, MNAzymes, a versatile new class of nucleic acid enzymes that can function as biosensors and molecular switches, J. Am. Chem. Soc., 2010, 132, 1051–1059 CrossRef CAS PubMed.
- T. Kuwabara, M. Warashina and K. Taira, Allosterically controllable ribozymes with biosensor functions, Curr. Opin. Chem. Biol., 2000, 4, 669–677 CrossRef CAS.
- N. K. Vaish, V. R. Jadhav, K. Kossen, C. Pasko, L. E. Andrews, J. A. McSwiggen, B. Polisky and S. D. Seiwert, Zeptomole detection of a viral nucleic acid using a target-activated ribozyme, RNA, 2003, 9, 1058–1072 CrossRef CAS.
- F. Wang, J. Elbaz, C. Teller and I. Willner, Amplified detection of DNA through an autocatalytic and catabolic DNAzyme-mediated process, Angew. Chem., Int. Ed., 2011, 50, 295–299 CrossRef CAS PubMed.
- F. Wang, J. Elbaz, R. Orbach, N. Magen and I. Willner, Amplified analysis of DNA by the autonomous assembly of polymers consisting of DNAzyme wires, J. Am. Chem. Soc., 2011, 133, 17149–17151 CrossRef CAS PubMed.
- K. Zagorovsky and W. C. W. Chan, A plasmonic DNAzyme strategy for point-of-care genetic detection of infectious pathogens, Angew. Chem., Int. Ed., 2013, 125, 3250–3253 CrossRef.
- E. Mokany, Y. L. Tan, S. M. Bone, C. J. Fuery and A. V. Todd, MNAzyme qPCR with superior multiplexing capacity, Clin. Chem., 2013, 59, 419–426 CAS.
- Y. V. Gerasimova, E. M. Cornett, E. Edwards, X. Su, K. H. Rohde and D. M. Kolpashchikov, Deoxyribozyme cascade for visual detection of bacterial RNA, ChemBioChem, 2013, 14, 2087–2090 CrossRef CAS PubMed.
- S. M. Bone and A. V. Todd, MNAzymes provide a universal mechanism for triggering DNAzyme synthesis cascades, Chem. Commun., 2014, 50, 13243–13246 RSC.
- A. Maruyama, M. Katoh, T. Ishihara and T. Akaike, Comb-type polycations effectively stabilize DNA triplex, Bioconjugate Chem., 1997, 8, 3–6 CrossRef CAS PubMed.
- A. Maruyama, H. Watanabe, A. Ferdous, M. Katoh, T. Ishihara and T. Akaike, Characterization of interpolyelectrolyte complexes between double-stranded DNA and polylysine comb-type copolymers having hydrophilic side chains, Bioconjugate Chem., 1998, 9, 292–299 CrossRef CAS PubMed.
- L. Wu, N. Shimada, A. Kano and A. Maruyama, Poly(l-lysine)-graft-dextran copolymer accelerates DNA hybridization by two orders, Soft Matter, 2008, 4, 744–747 RSC.
- W. J. Kim, T. Ishihara, T. Akaike and A. Maruyama, Comb-type cationic copolymer expedites DNA strand exchange while stabilizing DNA duplex, Chemistry, 2001, 7, 176–180 CrossRef CAS.
- W. J. Kim, T. Akaike and A. Maruyama, DNA strand exchange stimulated by spontaneous complex formation with cationic comb-type copolymer, J. Am. Chem. Soc., 2002, 124, 12676–12677 CrossRef CAS PubMed.
- W. J. Kim, Y. Sato, T. Akaike and A. Maruyama, Cationic comb-type copolymers for DNA analysis, Nat. Mater., 2003, 2, 815–820 CrossRef CAS PubMed.
- J. Gao, N. Shimada and A. Maruyama, Enhancement of deoxyribozyme activity by cationic copolymers, Biomater. Sci., 2015, 3, 308–316 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Comparison of effect of cofactors Mn and Mg ions to the MNAzyme reaction in the absence of PLL-g-Dex. Sequences and multiple-turnover reaction rates for the parent DNAzymes, Fig. 5B plotted with the reduced y-axis, and predicted melting temperatures (Tm) of duplexes having sequences of substrate- or target-binding domains of MNAzymes A and B. MNAzyme LoD determination. See DOI: 10.1039/c4bm00449c |
| This journal is © The Royal Society of Chemistry 2015 |




