Open Access Article
Open Access ArticleAn integrated carbon entrapped molecularly imprinted polymer (MIP) electrode for voltammetric detection of resveratrol in wine
S. M.
Mugo
*,
B. J.
Edmunds
,
D. J.
Berg
and
N. K.
Gill
Department of Physical Sciences (Chemistry), Grant MacEwan University, Edmonton, AB T5J 4S2, Canada. E-mail: mugos@macewan.ca; Fax: +1 7804975655; Tel: +1 7806333493
First published on 15th September 2015
Abstract
A carbon entrapped molecularly imprinted polymer (CEMIP) electrode has been demonstrated as a sensitive and selective voltammetric sensor for the in situ detection of resveratrol in red wine. Using differential pulse voltammetry (DPV), the CEMIP was compared to the carbon entrapped non-imprinted polymer (CENIP), with the resveratrol imprinted format found to be 12 times more sensitive for the detection of resveratrol. The CEMIP and CENIP had a detection limit of 20 and ∼100 μg L−1, respectively, with both electrodes giving good linear standard addition calibrations with R2 ≥ 0.99 for concentrations between 0.1 and 5 mg L−1, which is the usual occurrence range of resveratrol in wine. Compared to the conventional carbon MIP composite (CMIPC), the CEMIP platform was 2.7 orders of magnitude more sensitive, which is attributed to the better electron transfer and unhindered access of the analyte to the responsive sites within the imprinted polymer. The CMIPC was only ∼2.5 times more sensitive than the CNIPC. The %RSD for CEMIP and CMIPC for ∼5.0 mg L−1 of resveratrol in spiked wine was determined to be 3.2% and 5.1%, respectively.
Introduction
Chemical sensors such as ion selective electrodes (ISEs) are of particular interest in chemical analysis for they offer inexpensive, selective and rapid analysis of analytes amidst complex matrices, without the need for expensive chromatographic techniques.1–5 Of core importance in the ISEs is the study of membrane materials that give high selectivity necessary for a device with low detection limits. In general, selectivity is achieved by using materials that are inherently selective to an analyte (e.g. glass), integration of natural or synthetic ionophores in membranes, or immobilization of enzymes that react specifically to a substrate resulting in a product that can induce a measurable response.6–11 Numerous types of enzymes, such as glucose oxidase, cholesterol oxidase, β-galactosidase etc., have been immobilized on membranes for selective reaction and analysis of the corresponding species.8–10 While enzymes are very selective and preferable, they are expensive and there are only a few enzymes available that can act on analyte substrates of interest, thus limiting their applicability.Ionophores have filled the gap for impacting selectivity in the fabrication of chemical sensors. Instructively, most of the ionophores work based on the cavity entrapment of the analyte. Examples of common ionophores used for ISEs include membranes impregnated with: doped crystalline structure antibiotics (e.g. valinomycin), crown ethers, calixarenes, cyclodextrins, fullerenes etc.11–13 However, only few analytes have available corresponding selective ionophores, which limits their widespread applicability. Synthesis of some of the above ionophores can be intricate and thus quite expensive.
A new class of polymers being employed for chemical sensor applications are ‘smart’ polymers. The term ‘smart’ has been used to describe stimuli responsive hydrogels as well as molecularly imprinted polymers (MIPs).14,15 Fabrication of polymers for molecular recognition is a vast field of study with promise, due to the versatility and the many possibilities in polymer chemistry, especially in preparing selective membranes.15
This article will focus on the use of MIPs for the recognition of compounds of interest. MIPs refer to polymers in which monomers and crosslinkers are polymerized in the presence of a template. After polymerization, the template molecule is washed off, with the resulting polymer bearing cavities with size and shape mimicking the template molecule.15
Unlike enzymes and most ionophores, MIPs are inexpensive, easy to prepare, physically and chemically stable and versatile to accommodate different types of templates. In general, MIPs can be in the bulk form where the recognition cavities are distributed in a three dimensional (3D) form.16–21 On the other hand, MIPs can be fashioned and supported by a platform, such as silica, magnetic and polystyrene microspheres, and tubular formats or on glassy carbon electrodes, where a ‘2D’ MIP film is formed, an approach referred to as surface imprinting.22–30 The MIP film affords improved analyte binding kinetics and ensures that the cavities are easily accessible. There are numerous methods described in the literature for surface imprinting such as electropolymerization,22–24 atom transfer radical polymerization (ATRP)25,26 reversible addition–fragmentation chain transfer (RAFT) polymerization,27 and a self-assembly homo-polymerization method.28–31 Both RAFT and ATRP methods are in general time consuming and involve a multi-reaction process that requires grafting initiators on the surface of the support, to localize the site for polymerization. In addition, the grafting of the initiators in some cases is never homogeneous, hence resulting in an uneven polymer film coating.26In situ polymerization methods are more efficient and versatile, but have only been demonstrated in a limited number of platforms.29–31
In this manuscript, a facile approach for making resveratrol MIP thin films by entrapment of carbon beads is demonstrated and employed as an integrated carbon modified electrode for the voltammetric detection of resveratrol in wine. To demonstrate the applicability of the resveratrol carbon entrapped molecularly imprinted polymer (CEMIP), the device has been employed for the analysis of resveratrol in wine.
Resveratrol is a phytoalexin that is produced in several plant (such as mulberries, peanuts, and grapes and hence wines) species in response to environmental stress. Resveratrol has several biological and pharmacological effects including: anticancer activity, cardioprotective activity, antioxidant activity, antiinflammatory activity etc.32 Rapid methods for the quantification of resveratrol in wines are especially of interest, as current methods of analysis such as HPLC with fluorescence, chemiluminescence or UV detection and silyl derivatization in tandem with GC-MS are lengthy.33–38
The resveratrol based CEMIP was prepared by the use of a carbon microsphere core entrapped with polymer from the polymerization of methacrylic acid (MAA) as the monomer, ethylene glycol dimethacrylate (EGDMA) as the crosslinker, resveratrol as the template and acetonitrile as the porogenic solvent. The CEMIP electrode platform performance was compared to that of the conventional carbon MIP composite (CMIPC) electrode, where carbon/MIP polymers/eicosane (binder) are mixed and packed in micropipette tips. Similar CMIPC modified electrodes have been demonstrated in the literature and proved selective for the detection of various compounds such as rivastigmine, levamisole hydrochloride, famciclovir, rutin, promethazine etc.16–21 The non-imprinted counterparts of the devices were also fashioned and compared with the MIP formats. Differential pulse voltammetry (DPV) was used as a resveratrol detection method mode, while cyclic voltammetry (CV) was used to investigate the electron transfer efficiency of the fabricated graphite modified electrodes.
Experimental procedures
Materials
All aqueous solutions were prepared using >18 MΩ Milli-Q water (Millipore, Bedford, MA, USA). Resveratrol, 4,4′-azobis(4-cyanovaleric acid) (ACVA), methacrylic acid (MAA), ethylene glycol dimethacrylate (EGDMA), potassium ferricyanate (K3[Fe(CN)6]), KCl, ethanol, carbon microspheres (2–12 μm), Supel™-Select HLB (60 mg) cartridge, eicosane, glacial acetic acid, N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA), and sodium acetate were obtained from Sigma Aldrich, Oakville, Ontario, Canada. Platinum wire (0.5 mm diameter, 99.997% purity) was purchased from Alfa Aesar, Ward Hill, MA, USA. Fused silica melting point capillaries, 1.5–1.8 × 90 mm, were purchased from Kimble Chase (Vineland, NJ, USA). 1–200 μL Eppendorf micropipette tips were obtained from Fisher Scientific, Ottawa, Canada.MIP & NIP synthesis and carbon MIP composite (CMIPC) and carbon NIP composite (CNIPC) preparation
The synthesis of the MIP and NIP was adapted from Arvand et al.16 The prepolymer mixture consisted of 0.252 mmol (57 mg) of resveratrol, 1 mmol (85 μL) of MAA (monomer), 5 mmol (940 μL) of EGDMA (crosslinker), 15 mg of ACVA (initiator) and 5 mL of acetonitrile. The mixture was thoroughly mixed and allowed to undergo polymerization overnight in an oven at 70 °C. The imprinted polymer matrix was washed five times by ultrasonication with 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 ethanol
10 ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetic acid, to remove the resveratrol template. To confirm that there was no trace of resveratrol left, a fluorescence spectrometer was used. Following the MIP wash, the polymer was dried in an oven overnight at 70 °C. The NIP was fabricated by the same process as that for the MIP, except that there was no resveratrol template added.
acetic acid, to remove the resveratrol template. To confirm that there was no trace of resveratrol left, a fluorescence spectrometer was used. Following the MIP wash, the polymer was dried in an oven overnight at 70 °C. The NIP was fabricated by the same process as that for the MIP, except that there was no resveratrol template added.
The carbon MIP composite (CMIPC) and carbon NIP composite (CNIPC) electrode preparation was similarly adapted from Arvand et al.16 Briefly, the composite was prepared by mixing 15% of the MIP or NIP (78 mg), 15% eicosane as the binder (78 mg) and 70% carbon beads (363 mg) in a mortar and pestle until a homogeneous powder was obtained. The composite was tightly packed (3.0 cm packing) into the 1–200 μL micropipette tip. A platinum wire (0.5 mm diameter) was inserted into the composite packing for electrical connection.
Preparation of the carbon entrapped molecularly imprinted polymer (CEMIP) electrode
Both CEMIP and CENIP electrodes were prepared by integrating the MIP formation with the electrode preparation. As illustrated in Fig. 1A, this was achieved by tightly packing carbon in a poly(MAA-co-EGDMA) polymer monolith fritted micropipette tip. The MIP/NIP pre-polymer solution mixture as described above was infused using a Hamilton syringe pump on the carbon packed micropipette tip. Following the prepolymer infusion, a platinum wire was immediately inserted, the distal ends were plugged, and polymerization was allowed to occur overnight at 70 °C. The MIP film encapsulated the carbon beads holding them tightly in their housing. The non-polymerized materials and entrapped resveratrol were washed off by the infusion of 10 mL of 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 ethanol
10 ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetic acid at a flow rate of 50 μL min−1, which was found to be the appropriate solvent volume to wash off all the resveratrol, as confirmed by using a fluorimeter. After the wash, the polymer frit was cut off to expose the CEMIP cross-section for chemical sensing. Fig. 1B shows an inset picture of the miniaturized CEMIP electrode.
acetic acid at a flow rate of 50 μL min−1, which was found to be the appropriate solvent volume to wash off all the resveratrol, as confirmed by using a fluorimeter. After the wash, the polymer frit was cut off to expose the CEMIP cross-section for chemical sensing. Fig. 1B shows an inset picture of the miniaturized CEMIP electrode.
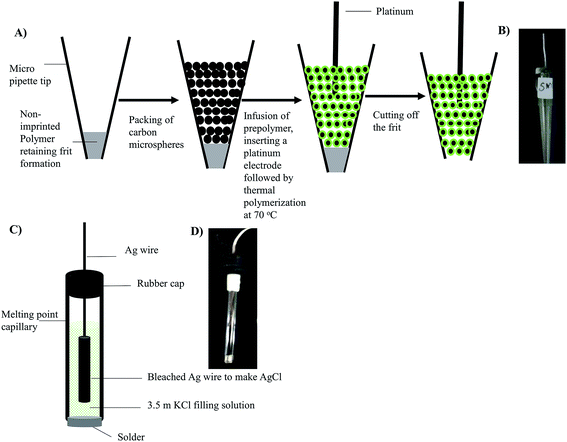 | ||
| Fig. 1 Schematic illustrating: (a) fabrication of CEMIP; (b) picture of CEMIP; (c) sketch of the fabricated Ag/AgCl reference electrode; (d) picture of the Ag/AgCl reference electrode. | ||
Reference electrode preparation
As illustrated in Fig. 1C, the miniaturized Ag/AgCl reference electrode was fabricated by using a melting point capillary tube (1.5 mm inner diameter, 3 cm long) from Kimble Chase (Vineland, NJ, USA) as the housing. The sealed end of the melting point capillary tube was broken open and a soldering metal of 0.5 cm length was inserted into the open end of the tube and soldered in place, providing a seal and for conductivity. The melting point capillary was filled with the 3.5 M KCl filling solution. To complete the reference electrode, an Ag wire that had been reacted with NaOCl for 30 min for obtaining an AgCl coat was inserted into the tube containing the electrolyte solution. To prevent the evaporation of the electrolyte over time, a rubber seal was used to cap the electrode. Fig. 1D shows an inset picture of the miniaturized fabricated Ag/AgCl.Electrochemical instrumentation and measurement
All electrochemical measurements were performed by using an Autolab potentiostat PGSTAT-101 with the fabricated Ag/AgCl as the reference electrode and platinum as an auxiliary electrode. To completely wash off the resveratrol template from the working electrodes between runs, cyclic voltammetry (CV) method was used. The CV cleaning method was as follows: the start and end potentials were set at −0.95 V and 0.95 V, respectively, with a scan rate of 50 mV s−1 and a step potential of 2.3 mV. A 0.2 M acetic acid/sodium acetate buffer (pH 5.5) with 0.1 M KCl was used as the electrolyte cleaning solution. For complete cleaning of the CEMIP electrodes, 10 cycles were used, with a change of wash buffer every 2 cycles. To ascertain that no trace of resveratrol was present in the wash solution, a fluorescence spectrometer was used.To confirm that the modified working electrodes and the fabricated Ag/AgCl reference electrode could afford an effective electron transfer, 5 mM K3[Fe(CN)6] in 0.1 M KCl was analyzed by CV, set at the same parameters described above. For quantitative analysis of resveratrol, differential pulse voltammetry (DPV) was used. For DPV, the start and end potentials were similarly set at −0.95 V and 0.95 V, respectively, with step potential at 2.3 mV, modulation amplitude at 25 mV, modulation time at 50 ms and interval time at 500 ms.
Resveratrol detection in standards and real samples
To confirm that the different modified resveratrol imprinted working electrodes were responsive to resveratrol, and to clearly confirm the redox peaks of interest, a 10 mg L−1 resveratrol standard prepared in 12% ethanol and 0.1 M KCl was analyzed. To confirm that the working electrodes could be used in real samples, a standard addition calibration approach was employed. The standard addition standard solutions were prepared by spiking 50 mg L−1 resveratrol standard in Barefoot red wine to make final spiked resveratrol concentrations of 0.00 (unspiked wine), 0.5, 1.0, and 5.0 mg L−1. To each standard, KCl salt was added to the final concentration of 0.1 M to ensure good conductivity. A 4 mL vial with 3 holes drilled on the cork for the insertion of electrodes was used as the electrochemical cell. Only 2 mL of the sample standard was used per run.Validation by the classic GC-MS method
To validate the voltammetric sensors developed, GC-MS (Agilent 6890N GC with 5975C MSD) was used to analyze the resveratrol spiked wine standards. Briefly, a Supel™-Select HLB (60 mg) cartridge was loaded with 2 mL of the standard solution. The cartridge was washed twice with 2 mL D.I. water, and then eluted with 1 mL of acetonitrile. The elution was repeated three times and the eluate evaporated to dryness with a gentle stream of nitrogen. The dried extract was reconstituted with 1 mL acetonitrile; 50 μL BSTFA added silylation was allowed to ensue for 1 h at 60 °C. 1 μL of sample aliquot was injected into the GC-MS, with the oven temperature increasing from 60–300 °C at 10 °C min−1. An Agilent HP-5ms column (30 m × 0.25 m × 0.25 m) was used.Results and discussion
To evaluate the morphology of the CEMIP and CMIPC electrodes, a JEOL 6301F (Field Emission Scanning Electron Microscope) was employed. Fig. 2A shows the SEM image of carbon beads, while Fig. 2B shows the SEM image of a cross-section of the CMIPC electrode. Clearly the resveratrol imprinted polymers were distributed around the carbon beads in the CMIPC format. The MIP microspheres (estimated to be ∼10 μm) are homogeneously integrated and distributed among the carbon microspheres, with the eicosane layer binding them into a monolithic block, with the latter acting as a binder to anchor the composite in place. Fig. 2C and D show the SEM image of a CEMIP electrode, where the carbon microspheres are entrapped by a homogeneous thin resveratrol MIP film. While the MIP film thickness has not been estimated due to the size heterogeneity of the carbon microspheres, it is clear that the imprinted polymer web holds the carbon in place and thus does not need a binder.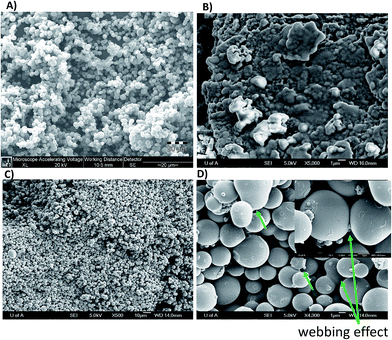 | ||
| Fig. 2 SEM images of (a) carbon microspheres; (b) CMIPC electrode morphology; (c) and (d) CEMIP electrode morphology. | ||
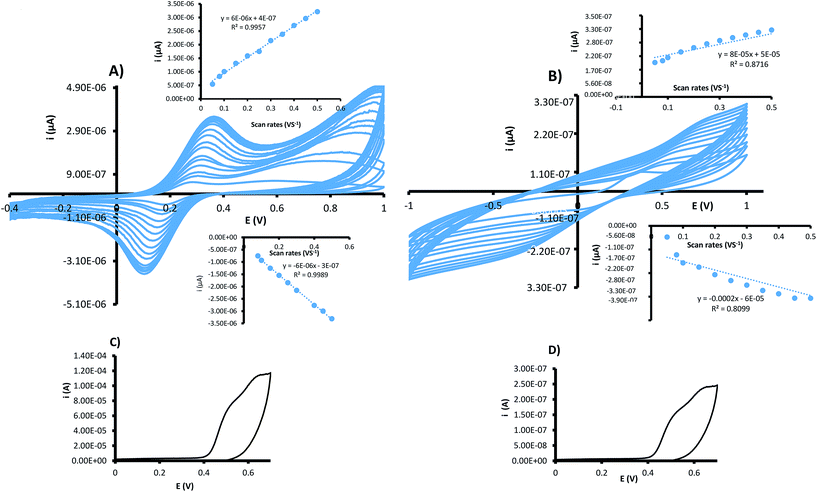
To assess the performance of the CMIPC and CEMIP electrode designs for electron transfer, 5 mM K3[Fe(CN)6], a reversible redox species, was analyzed by CV. Fig. 3A and B show the resulting voltammograms of the CEMIP and CMPIC at different scan rates (ranging from 0.05–0.5 V s−1) for the 5 mM K3[Fe(CN)6]. The CEMIP design resulted in better electron transfer with clear cathodic and anodic peaks for the ferricyanide solution. The CMIPC voltammograms have very broad anodic and cathodic peaks, suggesting hindered electron transfer due to the 3D structure of the non-conductive MIP as well as the presence of the eicosane binder. Comparing cathodic and anodic currents in Fig. 3A and B, the CEMIP was found to be ∼9 times more effective in electron transfer.
A common approach to evaluate electrochemical processes is by evaluating current as a function of scan rate. The inset in Fig. 3A shows a linear relationship of current as a function of scan rate, indicating a surface controlled electrode process. This corresponds with the CEMIP design where the imprinted polymer film envelops the conductive carbon. As for the CMIPC, the relationship between current as a function of scan rate deviates from linearity suggesting a less ideal surface electrode process.16,19
The obtained results are not surprising as the CEMIP consists of a thin MIP film; its design precludes the need for a binder (insulator) and thus is a much better electron conductor. In addition, the MIP film encapsulates the conductive carbon, webbing it to a monolithic block and also rigidly holding the platinum electrode in place, thus making the CEMIP design an evidently more robust and sensitive platform.
Cyclic voltammetry analysis of 10 mg L−1 resveratrol standard using the 2 carbon modified electrodes similarly showed (Fig. 3C and D) a high electron transfer for the CEMIP electrode.
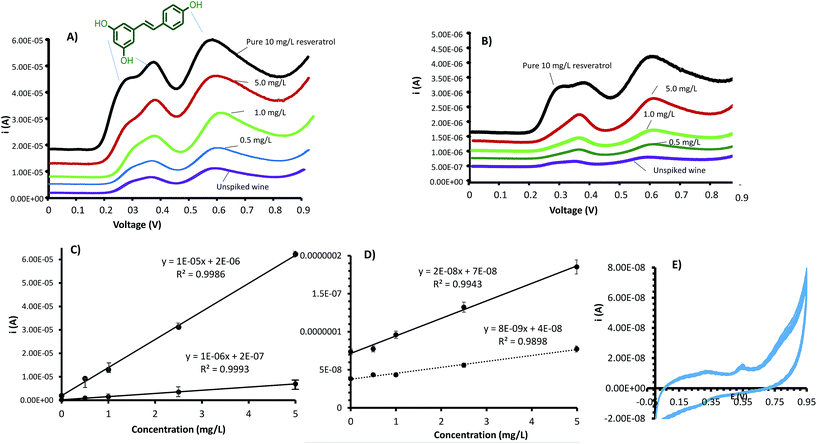
For quantitative investigation of resveratrol in wine, DPV was employed. Fig. 4A shows the DPV trace of 10 mg L−1 resveratrol standard, with three peaks corresponding to the three hydroxyl groups discriminated, clearly illustrating the power of DPV in functional group speciation. However, only the hydroxyl groups on different benzene rings are clearly distinguishable especially at a lower concentration. The hydroxyl groups on the same benzene rings are observed at ∼0.5 V as a shoulder separation at higher concentrations, but overlap at lower concentrations. The peak at ∼0.7 V corresponds to the single hydroxyl group in the benzene ring. By spiking different concentrations of resveratrol standard into a red wine sample, the voltammograms shown in Fig. 4A and B were obtained for CEMIP and CENIP, respectively. It was plausible to employ the standard addition calibration method for the testing, to simulate a real world sample, where matrix effects are corrected for. Both the peaks at ∼0.5 V and 0.7 V resulted in comparable linear calibrations with the latter shown in this article. To obtain the analyte signal for use in the calibration, the maximum current was subtracted from the baseline current and plotted as a function of concentration.
Fig. 4C shows the standard addition calibration plots obtained for CEMIP and CENIP electrodes. As evident in the calibration sensitivity plot, the CEMIP was about 12 times more sensitive than the non-imprinted entrapped electrode, attesting to the resveratrol selectivity, impacted by imprinting. Both the electrodes gave good linear standard addition calibrations with R2 ≥ 0.99 for concentrations between 0.1 and 5 mg L−1. The selectivity of the CEMIP electrode is in accordance with the linear standard addition calibration, as resveratrol is discriminated in the midst of a plethora of other compounds present in wine.
To compare the superiority of CEMIP as a more selective and sensitive platform to CMIPC electrodes, Fig. 4D shows the calibration plots obtained for CMIPC and CNIPC electrodes. As attested by the calibration sensitivity, CMIPC was found to be 2.5 times more sensitive than the non-imprinted counterpart. Comparing the CMIPC to the CEMIP, the latter was found to be ∼2.7 orders of magnitude more sensitive, which is clearly attributed to the MIP film advantage, where the resveratrol cavities are more accessible for selective extraction as well as better electron transfer due to the higher conductivity.
In general, the CMIPC was also found to be a selective platform for resveratrol, but inferior to the CEMIP due to the 3D format of the MIP. Therefore the demonstrated simplified approach for the formation of surface imprinted films on the carbon microspheres could be useful for the development of more sensitive electrochemical sensors to replace the routinely used carbon-imprinted polymer composite methods. Even in cases where thin layer imprinted polymers have been employed, a multistep fabrication is needed, where the initiators must be grafted on the surface of the microspheres being encapsulated.22–30
The 2 electrode design reproducibility and recoveries were evaluated by analysis of wine spiked with 5 mg L−1 of resveratrol as described earlier. Four different electrodes prepared under the same conditions were used to analyze the standard. Each electrode was reused four times with electrochemical cleaning between runs. As shown in Table 1, ∼97% and 89% were determined to be the percent recoveries for CEMIP and CMIPC respectively. The %RSD for the CMIPC and CEMIP was 5.1% and 3.2%, respectively, indicating good sensor to sensor reproducibility for both, but with superior repeatability for the CEMIP. The CMIPC reproducibility and recoveries are comparable to other similar electrodes reported in the literature for a similar class of compounds.16,17,19 On the other hand, even though the CENIP and CNIPC were reproducible, their % recoveries were only around 60%, which attests to the importance of imprinting in impacting selectivity. The memory effect was greatly alleviated by the electrochemical cleaning method and hence the same electrode can quickly be used to analyze multiple samples rapidly. The memory effect would be expected to be especially minimal for the CEMIP platforms as attested by a better %RSD of ∼3%. The effectiveness of electrochemical cleaning between runs was also confirmed by viewing the CV profile obtained by running the electrolyte cleaning solution, which indicated no resveratrol peaks after multiple CV cycles as illustrated in Fig. 4E.
| Electrode design | Measured resveratrol (n = 4) in mg L−1 | Recovery% | %RSD |
|---|---|---|---|
| CEMIP | 4.84 | 96.8 | 3.2% |
| CENIP | 3.13 | 62.6 | 3.4% |
| CMIPC | 4.47 | 89.4 | 5.1% |
| CNIPC | 2.99 | 60.0 | 6.9% |
| GC-MS | 5.11 | 102.2% | 2.2% |
Based on the signal of the baseline current, the CEMIP and CENIP detection limits were calculated to be 20 and ∼100 μg L−1, respectively.
While the detection limits are higher than those reported by LC-UV (∼3 μg L−1) and GC/MS, the detection limits are low enough for the detection of resveratrol in wines and other beverages.33–38 Notably, this work demonstrates that CEMIP with DPV mode is a valid, rapid, and inexpensive method, and could be employed in lieu of the conventional methods which are lengthy due to the separation and derivatization demands. It is to be noted that for low concentrations of resveratrol, the CEMIP could be employed as a selective preconcentration platform analogous to a solid extraction platform integrated with a working electrode.
The concentration of resveratrol in the brand of wine used was determined to be 0.35 ± 0.02 mg L−1 by using the CEMIP and 0.45 ± 0.08 mg L−1 by using the CMIPC after averaging 5 repeats. It should be noted that the five runs were conducted with the same electrode with electrochemical cleaning between the runs. The CEMIP had better precision compared to the CMPIC, which attests to the MIP film advantage. To validate the MIP voltammetric sensors by a standard technique, GC-MS was used to analyze the same brand of wine resulting in 0.37 ± 0.02 mg L−1, which closely matches those obtained by using the CEMIP voltammetric sensor.
Overall, the results were within the range of commonly observed resveratrol levels in wines using the mainstream techniques.33–38 It must be clarified that the wine sample was obtained from an opened bottle, and as such does not necessarily confirm the determined resveratrol concentrations to be the true concentrations in a new unopened wine from this brand.
Conclusion
The resveratrol base carbon entrapped molecularly imprinted platform has been demonstrated as an effective platform for the selective voltammetric detection of resveratrol in wine. The higher sensitivity of the CEMIP design compared to that of the conventional carbon MIP composite (CMIP) electrode could be attributed to the accessibility of the resveratrol cavities in the film morphology for the former. The CEMIP modified electrode design demonstrates an easy approach for integrating the conductive material and the recognition material (MIP) layer on the same platform with ease. Due to the thin layer design of the CEMIP, the electron transfer was found to be more efficient compared to the CMIP counterpart. In general the molecularly imprinted polymers impacted a higher selectivity compared to non-imprinted polymers, thus legitimizing their use as inexpensive synthetic ionophores, useful for the facile fabrication of chemical sensors. While NIP is generally selective, the CENIP design could still be useful as an integrated modified electrode platform for in general preconcentration of chemical entities close to the electrode surface. The NIP film can be used to tailor the hydrophobicity and hydrophilicity for the preconcentration of a class of analytes, as an aspect that could be useful for electrochemical analysis. The versatility of the fabrication of the CEMIP and the ease of replication for the use of other chemical entities of interest make the technology useful for widespread applicability.Acknowledgements
We acknowledge MacEwan University Department of Physical Sciences for reagents and instrumentation needed to carry out this work. The continued funding from MacEwan Research is also acknowledged.Notes and references
- J. Wang, Trends Anal. Chem., 2002, 21, 226–232, DOI:0165-9936/03
.
- F. J. Del Campo, Electrochem. Commun., 2014, 45, 91–94, DOI:10.1016/j.elecom.2014.05.013
.
- V. Gubala, L. F. Harris, A. J. Ricco, M. X. Tan and D. E. Williams, Anal. Chem., 2012, 84, 487–515, DOI:10.1021/ac2030199
.
- A. Escarpa, Chem. Rec., 2012, 12, 72–91, DOI:10.1002/tcr.201100033
.
- S. T. Mensah, Y. Gonzalez, P. Calvo-Marzal and K. Y. Chumbimuni-Torres, Anal. Chem., 2014, 86, 7269–7273, DOI:10.1021/ac501470p
.
- J. Langmaier, A. Trojanek and S. Zdenek, Electroanalysis, 2009, 21, 1977–1983, DOI:10.1002/elan.20094623
.
- J. Langmaier, J. Olsak, E. Samcova, Z. Samec and A. Trojanek, Electroanalysis, 2006, 18, 1329–1338, DOI:10.1002/elan.2006.3533
.
- Y. Lin, F. Lu, Y. Tu and Z. Ren, Nano Lett., 2004, 4, 191–195, DOI:10.1021/nl0347233
.
- D.-W. Zhang, J.-X. Liu, J. Nie, Y.-L. Zhou and X.-X. Zhang, Anal. Chem., 2013, 85, 2032–2036, DOI:10.1021/ac303223u
.
- M. I. Prodromidis and M. I. Karayannis, Electroanalysis, 2002, 14, 241–261, DOI:10.1002/1521-4109(200202)14:4<241::AID-elan241>3.0.CO;2-P
.
- P. Buhlmann, E. Pretsch and E. Bakker, Chem. Rev., 1998, 98, 1593–1688, DOI:10.1021/cr970113+
.
- X. Yang, D. Hibbert and P. Alexander, Anal. Chem. Acta, 1998, 372, 387–398, DOI:10.1016/S0003-2670(98)00382-1
.
- K. O’Connor, D. W. M. Arrigan and G. Svehla, Electroanalysis, 1995, 7, 205–215, DOI:10.1002/elan.1140070302
.
- I. Tokarev and S. Minko, Soft Matter, 2009, 5, 511–524, 10.1039/b813827c
.
- L. Ye and K. Mosbach, Chem. Mater., 2008, 20, 859–868, DOI:10.1021/cm703190w
.
- M. Arvand and P. Fallahi, Sens. Actuators, B, 2013, 188, 797–805, DOI:10.1016/j.snb.2013.07.092
.
- S. Sadeghi and A. Motaharian, Mater. Sci. Eng., C, 2013, 33, 4884–4891, DOI:10.1016/j.msec.2013.08.001
.
- M. Arvand and H. A. Samie, Drug Test. Anal., 2013, 5, 461–467, DOI:10.1002/dta.371
.
- B. Rezaei, N. Majidi, A. A. Ensafi and H. Karimi-Maleh, Anal. Methods, 2011, 3, 2510–2516, 10.1039/c1ay05271c
.
- N. A. El Gohary, A. Madbouly, R. M. El Nashar and B. Mizaikoff, Biosens. Bioelectron., 2015, 65, 108–114, DOI:10.1016/j.bios.2014
.
- T. Alizadeh and M. Akhoundian, Electrochim. Acta, 2010, 55, 5867–5873, DOI:10.1016/j.electacta.2010.05.037
.
- X. Zhang, Y. Peng, J. Bai, B. Ning, S. Sun, X. Hong, Y. Liu, Y. Liu and Z. Gao, Sens. Actuators, B, 2014, 200, 69–75, DOI:10.1016/j.snb.2014.04.028
.
- A.-E. Radi, A.-E. El-Naggar and H. M. Nassef, Anal. Methods, 2014, 6, 7967–7972, 10.1039/c4ay01320d
.
- H. da Silva, J. G. Pacheco, J. M. C. S. Magalhães, S. Viswanathan and C. Delerue-Matos, Biosens. Bioelectron., 2014, 52, 56–61, DOI:10.1016/j.bios.2013.08.035
.
- D. Gao, Z. Zhang, M. Wu, C. Xie, G. Guan and D. Wang, J. Am. Chem. Soc., 2007, 129, 7859–7866, DOI:10.1021/ja070975k
.
- L. Schweitz, Anal. Chem., 2002, 74, 1192–1196, DOI:10.1021/ac0156520
.
- J. Li, R. Dong, X. Wang, H. Xiong, S. Xu, D. Shen, X. Song and L. Chen, RSC Adv., 2015, 5, 10611–10618, 10.1039/c4ra11177j
.
- Q. Zhao, H. Li, Y. Xu, F. Zhang, J. Zhao, L. Wang, J. Hou, H. Ding, Y. Li, H. Jin and L. Ding, J. Chromatogr. A, 2015, 1376, 26–34, DOI:10.1016/j.chroma.2014
.
- J. Dai, Z. Zhou, C. Zhao, X. Wei, X. Dai, L. Gao, Z. Cao and Y. Yan, Ind. Eng. Chem. Res., 2014, 53, 7157–7166, DOI:10.1021/ie404140y
.
- A. Afkhami, H. Ghaedi, T. Madrakian, M. Ahmadi and H. Mahmood-Kashani, Biosens. Bioelectron., 2013, 44, 34–40, DOI:10.1016/j.bios.2012.11.030
.
- P. Ma, Z. Zhou, W. Yang, B. Tang, H. Liu, W. Xu and W. Huang, J. Appl. Polym. Sci., 2015, 132, 1–12, DOI:10.1002/app.41769
.
- J. Baur and D. Sinclair, Nat. Rev. Drug Discovery, 2006, 5, 493–506, DOI:10.1038/nrd2060
.
- I. Kolouchova-Hanzlikova, K. Melzoch, V. Filip and J. Smidrkal, Food Chem., 2003, 87, 151–158, DOI:10.1016/j.foodchem.2004.01.028
.
- D. A. Rodriguez, T. G. Diaz and I. D. Meras, Food Chem., 2010, 122, 1320–1326, DOI:10.1016/j.foodchem.2010.03.098
.
- J. Zhou, C. Hua, G. Wan, H. Xu, Y. Pan and C. Duan, Food Chem., 2004, 88, 613–620, DOI:10.1016/j.foodchem.2004.05.003
.
- J. L. Cacho, N. Campillo, P. Viñas and M. Hernández-Córdoba, J. Chromatogr. A, 2013, 1315, 21–27, DOI:10.1016/j.chroma.2013
.
- F. Dias, M. F. Silva and J. M. David, Food Anal. Methods, 2013, 6, 963–968, DOI:10.1007/s12161-012-9507-2
.
- C. H. Lin and Y. H. Chen, Electrophoresis, 2001, 22, 2574–2579, DOI:10.1002/1522-2683(200107)22:12<2574::AID-ELPS2574>3.0.CO;2-M
.
| This journal is © The Royal Society of Chemistry 2015 |