Electro-oxidation of captopril at a gold electrode and its determination in pharmaceuticals and human fluids
Abstract
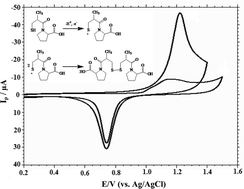
The electrochemical oxidation of captopril has been investigated by cyclic, linear sweep and differential pulse voltammetry in different pH ranges at a gold electrode. Captopril undergoes one electron and one proton change with an adsorption-controlled process. Effects of the anodic peak potential (Ep), anodic peak current (Ipa) and heterogeneous rate constant (k0) have been discussed. The effect of surfactants was also studied. The oxidation peak corresponds to the thiol and a probable mechanism was proposed. According to the linear relationship between the peak current and the captopril concentration, a differential-pulse voltammetric method for the quantitative determination of captopril was developed. The linear response was obtained in the range of 0.033–2.4 μM with a detection limit of 1.97 × 10−8 M with good selectivity and sensitivity. Furthermore, the proposed method was applied to the in vitro determination of captopril in pharmaceutical samples, spiked human urine and plasma adopting the differential pulse voltammetric technique for clinical research.

 Please wait while we load your content...
Please wait while we load your content...