Open Access Article
Open Access ArticleMulti-walled carbon nanotubes: innovative sorbents for pre-concentration of polychlorinated biphenyls in aqueous environments
Elizabeth N.
Ndunda
and
Boris
Mizaikoff
*
Institute of Analytical and Bioanalytical Chemistry, University of Ulm, Albert-Einstein-Allee 11, 89081 Ulm, Germany. E-mail: boris.mizaikoff@uni-ulm.de; Fax: +49-731-50-22763; Tel: +49-731-5022750
First published on 21st July 2015
Abstract
Carbon nanotubes (CNTs) have demonstrated outstanding chemical and mechanical stability, electrical properties, and strong interactions with aromatic compounds owing to the π-electron system on the graphene sheets. Taking advantage of these unique properties, we have developed a fully validated sample pre-concentration technique for determination of polychlorinated biphenyls (PCBs) in aqueous environments using gas chromatography combined with a micro-cell electron capture detector (GC-μECD). The optimized method using pristine MWCNTs gave recoveries in the range of 46.0–92.5%, 51.4–91.5%, 48.7–77.8% for tap water, river water, and lake water, respectively. Compared to conventional C18 adsorbent and oxidized MWCNTs (oMWCNTs), pristine MWCNTs provided the best recoveries, thereby confirming that MWCNTs are excellent alternatives for C18, with the ability to achieve high performance. The developed protocol achieved method detection limits in the range of 0.002–0.011 μg L−1 and relative standard deviation (RSD) < 15.5%.
1. Introduction
Carbon nanotubes (CNTs) are materials of increasing interest since their discovery by Iijima in 1991.1 The two forms of CNTs are the single-walled carbon nanotubes (SWCNTs) and the multi-walled carbon nanotubes (MWCNTs), which are formed from either a single roll or several cylindrical shells of graphene sheet, respectively. CNTs are characterized by their extraordinary chemical and mechanical stability, unique electrical properties,2–4 and strong interactions with aromatic constituents due to the delocalized π electron system.5However, especially in aqueous environments their application has been limited due to their strong van der Waals forces leading to agglomeration. Past studies have indicated that surface modification via covalent and non-covalent interactions improves the solubility of CNTs in organic and aqueous media.2,3 The non-covalent modification is a physical process, which entails wrapping of polymers around the surface of the CNTs.6,7 In contrast, covalent modification usually involves the introduction of hydroxyl groups or carboxyl groups at the surface of the CNTs, which then provides a chemical architecture for further functionalization of the surface.2
Surface-modified CNTs have therefore been used in biosensors,8 electrochemical sensors,9 diagnostics imaging,10,11 drug-delivery systems,12 nanocomposites,13 and in general separation sciences.14 Furthermore, CNTs have been proposed as new generation of adsorbents in solid-phase extraction (SPE), facilitating the analysis of environmental pollutants.15 Particularly, CNTs have been applied as adsorbents in SPE for quantification of polycyclic aromatic compounds,16 phthalate esters,17 polyhalogenated compounds,18 and tetracyclines.19 Since studies on the adsorption of dioxins at CNTs indicated that they are excellent materials for the removal of dioxins,20 it is anticipated that CNTs should be suitable materials for pre-concentrating polychlorinated biphenyls (PCBs) from aqueous samples.
PCBs are compounds that were majorly used as dielectric fluids in capacitors and transformers during their commercial production between the 1930s and the 1970s, resulting to subsequent releases into the environment. Hence, they have been detected in soil,21,22 sediments,23–25 water,26 air,27 and biota;28 even in areas without any commercial production, PCBs are found to date. Consequently, their wide environmental distribution is directly attributed to their stability, their long-range transport, and their persistence ranking them among the most prevalent contaminants in environmental matrices.29 Therefore, monitoring of these compounds is of substantial relevance for tracking either contemporary releases or for developing appropriate strategies towards their complete remediation and elimination from the environment, as is the long term goal of the Stockholm convention on persistent organic pollutants (POPs).
However, their determination remains challenged due to their occurrence at trace-to-ultra-trace levels, and in complex matrices, thus the need for a pre-treatment step to facilitate detection at such low concentrations. Conventional sample preparation steps usually include liquid–liquid extraction (LLE) followed by a clean-up procedure or solid phase extraction (SPE) based on C18 sorbents. While LLE is demanding in terms of the volume of solvent required and the extraction time using, e.g., separatory funnels, C18-SPE is limited by the non-selective enrichment of various constituents leading to co-elution, which then may interfere with the quantitative determination.
MWCNTs provide an attractive option based on their chemical properties serving as adsorbents for aromatic constituents. However, to date they are still not considered analytically or commercially viable alternatives to conventional C18 sorbents, and only few studies have reported on the preconcentration of PCBs, i.e., enriching PCBs from aqueous samples using magnetic MWCNTs grafted with a hydrophilic layer,30 and clean-up of fish extracts using magnetic molecularly imprinted polymers comprising MWCNTs as support material.31
Though modifications are necessary especially when dealing with complex matrices, the long procedures of synthesis and grafting are not only time consuming but the need for extra reagents makes it costly for continuous monitoring as is required for PCBs. Therefore, we report herein an affordable and fully validated MWCNTs-SPE protocol for determination of 6 indicator PCBs (i.e., IUPAC no. 28, 52, 101, 153, 138, 180) in aqueous environments using pristine MWCNTs. We also prove that pristine MWCNTs are excellent pre-concentration materials compared to their oxidized forms and conventional C18, thereby resulting in a ready-to-use SPE adsorbent.
2. Materials and methods
2.1 Chemicals
SPE cartridges (6 mL) and frits (20 μm porosity), pesticides grade methanol, n-hexane, and dichloromethane (DCM) were purchased from Carl Roth Chemicals (Karlsruhe, Germany). The two types of MWCNTs used were: MWCNTs with outer diameter 110–170 nm, length 5–9 μm, and purity > 90% carbon basis, and MWCNTs with outer diameter 10 nm, length 3–6 μm, and purity ≥ 98% carbon basis synthesized via catalytic chemical vapour decomposition (Sigma-Aldrich, Steinheim, Germany). PCB standard mixtures (no. 28, 52, 101, 138, 153, 180), PCB 15 & 209, and Supelclean™ LC-18 SPE cartridges (6 mL, 500 g, 51.7 μm, 490 m2 g−1) were purchased from Sigma-Aldrich (Steinheim, Germany). MWCNTs were dried at 120 °C for 2 h before use. Nitric acid (65%) and sulphuric acid (95–97%) were bought from Merck KGaA (Darmstadt, Germany). Water used in this study was purified using a Milli-Q filter system from Millipore (Billerica, USA). Nitrogen (99.999%) for gas chromatography was supplied by MTI IndustrieGase AG (Neu-Ulm, Germany).2.2 Instrumentation
Gas chromatography was performed using an Agilent 6890 (Agilent Technologies) system coupled to a micro-cell electron capture detector (GC-μECD) for all the analysis discussed herein. The column used for the separation of PCBs was a ZB5-MS capillary column of dimensions 30 m × 0.25 mm i.d. × 0.25 μm film thickness with a 1 m silica-coated deactivated guard column (0.32 mm i.d.), which was connected to the analytical column via a glass capillary connector. The two temperature programs applied were: (i) initial temperature of 60 °C (hold time 2 min), ramp at 20 °C min−1 to 260 °C (hold time 5 min) for PCB 15, and (ii) initial temperature of 60 °C (hold time 2 min), ramp at 15 °C min−1 to 210 °C (hold time 2 min), and final ramp at 15 °C min−1 to 275 °C (hold time 5 min) for the PCB mixtures. The detector temperature was set at 280 °C.1 μL of standards and samples was manually injected using the on-column injection mode. Nitrogen (purity > 99.999%) was used as both carrier gas at a flow rate of 2 mL min−1, and as the detector make-up at 30 mL min−1. Quantification was based on internal standard calibration using PCB 209, while the identification was performed by comparing the retention times with those of standards. Data was processed using the ChemStation software version A.03.08 supplied by Agilent Technologies.
2.3 Oxidation of MWCNTs
An amount of 0.5 g of pristine MWCNTs (10 nm) were placed in 100 mL flask, and 100 mL of H2SO4/HNO3 (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; v/v) was added. The mixture was sonicated at 40 °C for 4 h (60 W, 35 kHz) and the resultant oxidized MWCNTs (oMWCNTs) were diluted with 500 mL water, filtered under vacuum, and washed with ultrapure water until neutral pH; thereafter, they were air dried overnight.
1; v/v) was added. The mixture was sonicated at 40 °C for 4 h (60 W, 35 kHz) and the resultant oxidized MWCNTs (oMWCNTs) were diluted with 500 mL water, filtered under vacuum, and washed with ultrapure water until neutral pH; thereafter, they were air dried overnight.
2.4 Characterization of pristine MWCNTs and oMWCNTs
The surface morphology was investigated using a DualBeam Helios Nanolab 600 focused ion beam (FIB) – scanning electron microscopy (SEM) system (Hillsboro, OR, USA). Nitrogen adsorption–desorption experiments were performed using QuadraSorb Station SI from Quantachrome GmbH and Co. KG (Odelzhausen, Germany). Samples were degassed at 100 °C under vacuum for 3 h before data collection, and the specific surface area was calculated by the Brunauer–Emmett–Teller (BET) method. Studies on the oxygen-containing functional groups at the surface of MWCNTs were done by X-ray photoelectron spectroscopy (XPS).2.5 Validation of MWCNTs-SPE
One hundred milligrams of the MWCNTs was weighed into a 6 mL empty polypropylene cartridge with a frit (20 μm porosity) at the bottom. Aliquots of methanol were passed through the column to ensure tight packing of the adsorbent. Thereafter, a second layer of frit was added on top of the packed adsorbent. The packed column was then mounted onto the Visiprep™ SPE vacuum manifold, and conditioned with 6 mL of methanol followed by equilibration with 6 mL Milli-Q water. During these steps, the column was not allowed to dry. Spiked water samples were loaded onto the column followed by washing with 2 mL of methanol. Prior to elution, the column was dried for 15 min by drawing air through the device under full vacuum. The analytes were desorbed using 10 mL n-hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DCM (1
DCM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; v/v) into round bottomed flasks, and reduced to 0.5 mL using a rotary evaporator immersed in a water bath set at 30 °C. The eluate was then transferred into a glass vial, where the volume was further reduced to near dryness under a gentle flow of argon, and reconstituted in hexane into GC autosampler vials. 4 μL of PCB 209 (8.8 μg mL−1) was added before GC-μECD analysis. Conventional C18 SPE columns were subject to the same processing steps (i.e., conditioning and equilibration) as the MWCNT-SPE cartridges, however, were washed with 10% methanol in water.
1; v/v) into round bottomed flasks, and reduced to 0.5 mL using a rotary evaporator immersed in a water bath set at 30 °C. The eluate was then transferred into a glass vial, where the volume was further reduced to near dryness under a gentle flow of argon, and reconstituted in hexane into GC autosampler vials. 4 μL of PCB 209 (8.8 μg mL−1) was added before GC-μECD analysis. Conventional C18 SPE columns were subject to the same processing steps (i.e., conditioning and equilibration) as the MWCNT-SPE cartridges, however, were washed with 10% methanol in water.
2.6 Collection of water samples and analysis
Tap water was collected from the laboratory (Institute of Analytical and Bioanalytical Chemistry, University of Ulm, Germany). River water was obtained from the Danube river (Ulm), and lake water was collected from the Ludwigfelder See (Neu-Ulm). The water samples were collected into glass bottles, then filtered through 0.45 μm pore filters, and stored at 4 °C until analysis following the developed method.2.7 Adsorption capacity
Twenty milligrams of MWCNTs were packed into Eppendorf tubes, and 1 mL of 0.4 μg mL−1 PCB 15 in hexane was added and equilibrated for 4 h. The extent of adsorption was followed by analyzing the supernatant every 30 min. The tubes were centrifuged at 3000 rpm for 5 min, and the amount of PCB in the supernatant was determined by GC-μECD analysis. After establishing the time required to reach equilibrium, 20 mg MWCNTs were incubated with 1 mL of PCB 15 at concentrations ranging from 0.4–4.8 μg mL−1, and vortexed for 60 min. The amount of bound analyte was calculated using eqn (1) and binding characteristics determined by applying the binding data to Langmuir (2) and Freundlich (3) equations. | (1) |
 | (2) |
| qe = KfCen | (3) |
2.8 Quality control
The quality control measures included analysis of blank samples, rinsing of glassware before use, and the use of internal standards. The method performance was tested by determining accuracy, precision, linearity, and method detection limit (MDL). Standards were prepared from 10 μg mL−1 of PCB mixture stock standards by dilution with n-hexane. Quantification was based on the internal standard method with PCB 209 added to all samples and standards. The MDL was determined following the EPA method using blank samples (Milli-Q water) spiked at low concentrations (4-times the concentration providing a signal-noise-ratio of 3), and then taken through the validated analytical procedure.323. Results and discussion
3.1 Characterization of pristine MWCNTs and oMWCNTs
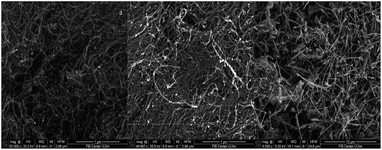
The SEM images revealed entangled fibres for both pristine MWCNTs and oMWCNTs. While oxidation is thought to affect both the structure and the physical properties of the MWCNTs, according to the SEM images the structure remained essentially intact, as no observable change in surface morphology were evident (Fig. 1a and b). MWCNTs (110–170 nm) revealed very short fibres and large diameters, as anticipated (Fig. 1c). Atomic force microscopy (AFM) studies have shown that there is an insignificant decrease in length following the oxidation process.33 Raman spectroscopy of the oxidized MWCNTs compared to pristine MWCNTs has indicated that the graphene structure remains intact despite the curtailing of the fibre lengths.34,35 However, the oxidation process induces defects (i.e., amorphous carbon) with the degree of deformation at the graphene sheets characterized by the IG/ID ratio, which is the ratio of the G-band (i.e., sp2 hybridized carbon), and D-band (i.e., sp3 hybridized carbon) intensities.5,36The increased dispersion of MWCNTs in water proved the successful oxidation of MWCNTs. Introduction of oxygen containing groups was also confirmed using XPS where increased intensity of oxygen (O1s) peak for the oMWCNTs (Fig. 2b), compared to the pristine MWCNTs (Fig. 2a) was observed. The total oxygen content is shown in Table 1. Specific surface areas determined via BET were 269.7, 244.8, and 17.4 m2 g−1 for the pristine MWCNTs (10 nm), oMWCNTs, and pristine MWCNTs (110–170 nm), respectively. Therefore, no pronounced decrease in surface area was observed due to the oxidation process; likewise, recent studies confirm minute changes or no evident change in specific surface area after oxidation.37–39
| Peak | Pristine MWCNTs | oMWCNTs |
|---|---|---|
| C1s | 98.0 | 91.69 |
| O1s | 2.0 | 8.31 |
3.2 Validation of MWCNTs-SPE
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DCM (1
DCM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) gave the best results (>70%) for all the PCBs congeners, and was therefore adopted for all further analyses using 10 mL of volume. CNTs have been reported to strongly interact with aromatic compounds;20 hence, the need for a strong elution solvent, which can efficiently and effectively disrupt the interactions with the CNT surface.
1) gave the best results (>70%) for all the PCBs congeners, and was therefore adopted for all further analyses using 10 mL of volume. CNTs have been reported to strongly interact with aromatic compounds;20 hence, the need for a strong elution solvent, which can efficiently and effectively disrupt the interactions with the CNT surface.
3.3 Analysis of real world water samples
The pH of the water samples was determined as 7.69 ± 0.02, 8.42 ± 0.03, and 8.12 ± 0.00 for tap water, river water, and lake water, respectively. Water samples were analyzed before spiking to ascertain the presence or absence of any PCBs; no PCBs were detected in any of the samples. Then, the samples were spiked at a level of 0.2 ng mL−1, and analyzed via the validated method. The recoveries ranged from 46.0–92.5% for tap water, 51.4–91.5% for river water, and 47.8–77.8% for lake water (Table 2). Five out of six congeners had recoveries within 70–120%, yet again, PCB 28 gave poor recoveries (<70%). PCB 28 has the lowest number of chlorine atoms and is therefore expected to be highly soluble in water in contrast to the other five PCBs studied herein.| Analyte | Tap water | River water | Lake water | |
|---|---|---|---|---|
| MWCNTs | oMWCNTs | MWCNTs | MWCNTs | |
| a STD is the standard deviation of triplicate analysis. | ||||
| PCB 28 | 46.0 ± 4.94 | 42.9 ± 1.40 | 51.4 ± 4.34 | 48.7 ± 7.57 |
| PCB 52 | 90.7 ± 4.23 | 54.4 ± 4.04 | 91.5 ± 2.30 | 77.7 ± 1.84 |
| PCB 101 | 90.1 ± 3.61 | 48.9 ± 4.11 | 87.0 ± 6.06 | 76.7 ± 3.39 |
| PCB 153 | 91.3 ± 3.48 | 46.6 ± 4.52 | 86.7 ± 6.01 | 77.8 ± 3.25 |
| PCB 138 | 92.5 ± 3.46 | 44.9 ± 4.14 | 91.1 ± 8.37 | 74.1 ± 1.77 |
| PCB 180 | 90.3 ± 5.19 | 43.4 ± 7.75 | 87.6 ± 6.92 | 76.5 ± 5.09 |
The aqueous solubility of PCB 28 is 0.16 mg L−1, compared to 0.03, 0.01, 0.001 for PCB 52, 101, and 153, respectively.42 It is therefore likely that PCB 28 already suffers from losses during the loading step. The same phenomenon was reported by Dahane et al.,43 where the most soluble constituent methomyl gave recoveries in the range of 43–60%, compared to less soluble pesticides such as diazinon, whose recovery was between 58 and 106%. C18 adsorbent, which is the conventional adsorbent material used for most SPE applications recorded markedly lower recoveries (33.9–67.7%). Poor performance, i.e., recoveries <60.0% for C18 cartridges, have been previously reported.44–46 C18 is known to retain non-polar compounds via hydrophobic forces; yet, MWCNTs are subject to additional π–π interactions, and therefore strongly retain aromatic constituents.47
3.4 Effect of surface oxidation on the recovery of PCBs
To determine the effect of surface oxidation of MWCNTs on the recovery of PCBs, spiked Milli-Q water was pre-concentrated using oMWCNTs. Reduced recoveries were evident compared to pristine MWCNTs (Table 2). It should be noted that although the dispersion of functionalized MWCNTs in water increased due to the increase in hydrophilicity, the performance decreased. Similarly, a decrease in recovery of pentachlorophenol,38 naphthalene,33 phenanthrene,48 toluene, ethylbenzene, xylene,49 and triclosan37 on oxidized MWCNTs as compared to pristine MWCNTs has been reported. The reason for the decreased performance of oxidized MWCNTs has been linked to the oxygen groups (i.e., carboxyl and hydroxyl groups), which are hypothesized to localize the electrons at the surface of the MWCNTs, thus, removing them from the π-electron system.50 In addition, the formation of water clusters due to hydrogen bonding between the water molecules and the oxidized MWCNT surface has been argued as a possible reason for the decrease in retention pertaining non-polar molecules.33 Therefore, the retention of water molecules rather than non-polar constituents appears highly favoured.33,51 Cho et al.,33 suggested that oxidation could also prevent non-polar molecules from accessing the non-oxidized sites within CNT structures.3.5 Effect of pH on the recovery of PCBs
Some studies have reported the isoelectric point of oxidized MWCNTs to be around pH 4.37,39 Taking this into consideration, at lower pH oxidized CNTs are protonated, and no opportunity of hydrogen bond formation with water is provided. At higher pH, CNTs are deprotonated, and therefore, chances of hydrogen bonds with surrounding water molecules increase leading to the formation of water clusters. To determine whether the recovery of PCBs is improved at lower pH, tap water was spiked at a pH of 1 (i.e., pH below the reported isoelectric point for oxidized MWCNTs). The recoveries ranged from 41.4 to 50.3%, which was within the same range of recoveries as the tap water at a pH of 7.69. Therefore, it may be concluded that the introduction of polar groups (i.e., surface oxygen) generally increases the polarity of MWCNTs, which in turn leads to reduced interaction with aromatic constituents, and thus, reduced adsorption capacities.52 These results are also backed by the earlier argument that oxygen-containing groups localize electrons at the surface of MWCNTs, thereby removing them from the π-electron system, which results in reduced adsorption.3.6 Adsorption isotherms
The Langmuir adsorption isotherm given by eqn (2), and the Freundlich adsorption isotherm given by eqn (3) were applied for the binding characterization of the pristine MWCNTs (10 nm). Freundlich isotherms assume a heterogeneous surface with adsorption sites of different energies, while Langmuir isotherms are based on a homogeneous surface interaction. MWCNTs indicated a heterogeneous surface (n = 0.71) with the adsorption favouring the Freundlich isotherm, as confirmed by a correlation coefficient >0.99 (Table 3). Other studies on the adsorption of 1,2-dichlorobenzene, 1,2,4-trichlorobenzene, 1,2,4,5-tetrachlorobenzene, and trihalomethanes on CNTs have reported n-values of 0.52–0.65, 0.64, 0.75, and 0.58–0.75, respectively.41,53,54| Langmuir | Freundlich | ||||||
|---|---|---|---|---|---|---|---|
| q m (μg g−1) | K L (mL μg−1) | R L | R 2 | K f (μg g−1) | n | R 2 | |
| PCB 15 | 555.6 | 0.09 | 0.97 | 0.889 | 54.1 | 0.711 | 0.9972 |
3.7 Analytical performance of the proposed method
The obtained MDL values ranged from 0.002–0.011 μg L−1 with PCB 180 and PCB 138 providing the lowest MDL value, and PCB 28 the highest (Table 4). The RSD of replicate analyses ranged from 2.21–14.6%. The obtained calibrations were linear across the investigated concentration range of 0.465–186 ng mL−1 for lower chlorinated PCBs, and 0.21–104 ng mL−1 for the higher chlorinated ones at coefficients of linearity >0.99 (Table 4).| PCB congener | Retention time (min) | Linear range (ng mL−1) | Regression equation | Linear response (r2) | Method detection limit MDL (μg L−1) |
|---|---|---|---|---|---|
| PCB 28 | 12.9 | 0.465–186 | y = 0.015x + 0.0217 | 0.9997 | 0.011 |
| PCB 52 | 13.6 | 0.465–186 | y = 0.0073x + 0.014 | 0.9998 | 0.008 |
| PCB 101 | 15.5 | 0.26–104 | y = 0.0102x + 0.0159 | 0.9996 | 0.004 |
| PCB 153 | 17.2 | 0.21–85 | y = 0.0153x + 0.0176 | 0.9997 | 0.002 |
| PCB 138 | 17.8 | 0.23–92 | y = 0.0254x + 0.0242 | 0.9987 | 0.004 |
| PCB 180 | 19.3 | 0.22–89 | y = 0.0279x + 0.0174 | 0.9998 | 0.002 |
4. Conclusions
A fully validated MWCNT-SPE protocol for the pre-concentration of PCBs in aqueous environments has been presented. Pristine MWCNTs revealed higher recoveries for the investigated PCBs compared to conventional C18 adsorbents and oMWCNTs, confirming that pristine MWCNTs can be applied for studies of PCBs in aqueous media. In addition, only 100 mg of MWCNTs were necessary compared to 500 mg for conventional sorbent, thereby suggesting not only more reliable, but potentially also more affordable method for SPE. Compared to C18, the cost of MWCNTs – at this small quantity – is already now slightly less, thus offering an excellent alternative to conventional sorbent matrices. The developed method shows detection limits way below the maximum contamination level of 0.5 μg L−1 for PCBs in drinking water (as set by US environmental protection agency (EPA)), therefore suggesting that the established analytical method may be readily implemented for monitoring of drinking water quality and safety, which is of substantial societal relevance. However, despite the results achieved herein, the developed protocol may only be applicable for pre-concentrating sufficiently chlorinated constituents, as more soluble (i.e., less chlorinated) constituents may be retained less due to the reduced interaction with MWCNTs.Acknowledgements
The authors thank G. Neusser and the Focused Ion Beam Center UUlm (Institute of Analytical and Bioanalytical Chemistry, University of Ulm) for the SEM images, C. Egger (Institute of Inorganic Chemistry II, University of Ulm) for the BET studies, and T. Diemant (Institute of Surface Chemistry and Catalysis, University of Ulm) for the XPS measurements. S. Gienger is appreciated for assistance with the gas chromatography experiments. E. Ndunda is grateful to the Ministry of Higher Education, Science and Technology (MOHEST) of Kenya in conjunction with the Deutscher Akademischer Austauschdienst (DAAD) for financial support.References
- S. Iijima, Nature, 1991, 354, 56–58 CrossRef CAS.
- K. Balasubramanian and M. Burghard, Small, 2005, 1, 180–192 CrossRef CAS PubMed.
- M. Valcárcel, B. M. Simonet, S. Cárdenas and B. Suárez, Anal. Bioanal. Chem., 2005, 382, 1783–1790 CrossRef PubMed.
- N. B. Singh and S. Agrawal, J. Sci. Med. Chem., 2013, 1, 1–8 CrossRef.
- R. Amade, S. Hussain, I. R. Ocaña and E. Bertran, J. Environ. Eng. Ecol. Sci., 2014, 3, 1–7 CrossRef CAS.
- A.-E. Hsiao, S.-Y. Tsai, M.-W. Hsu and S.-J. Chang, Nanoscale Res. Lett., 2012, 7, 2–5 CrossRef PubMed.
- A. Liu, I. Honma, M. Ichihara and H. Zhou, Nanotechnology, 2006, 17, 2845–2849 CrossRef CAS.
- C. Han, A. Doepke, W. Cho, V. Likodimos, A. A. de la Cruz, T. Back, W. R. Heineman, H. B. Halsall, V. N. Shanov, M. J. Schulz, P. Falaras and D. D. Dionysiou, Adv. Funct. Mater., 2013, 23, 1807–1816 CrossRef CAS.
- W. Guo, M. Geng, L. Zhou, S. Chao, R. Yang, H. An and H. Liu, Int. J. Electrochem. Sci., 2013, 8, 5369–5381 CAS.
- L. Gemma, G. Vidili, E. Venturelli, C. Ménard-moyon and M. Antonietta, PNAS, 2012, 109, 16612–16617 CrossRef PubMed.
- X. Shi, S. H. Wang, M. Shen, M. E. Antwerp, X. Chen, C. Li, E. J. Petersen, Q. Huang, W. J. Weber and J. R. Baker, Biomacromolecules, 2009, 10, 1744–1750 CrossRef CAS PubMed.
- A. M. A. Elhissi, W. Ahmed, I. U. Hassan, V. R. Dhanak and A. D’Emanuele, J. Drug Delivery, 2012, 2012, 1–10 CrossRef PubMed.
- A. May-Pat, F. Aviles, P. Toro, M. Yazdani-Pedram and J. Cauich-Rodriguez, eXPRESS Polym. Lett., 2011, 6, 96–106 CrossRef.
- Y. Chang, B. Bai, L. Du, T. Pang and Y. Fu, J. Chil. Chem. Soc., 2013, 58, 2209–2212 CrossRef CAS.
- B. Constantin, in Carbon Nanotubes, ed. J. M. Marulanda, InTech, Shanghai, 1st edn, 2010, pp. 524–542 Search PubMed.
- W.-D. Wang, Y.-M. Huang, W.-Q. Shu and J. Cao, J. Chromatogr. A, 2007, 1173, 27–36 CrossRef CAS PubMed.
- Y.-Q. Cai, G.-B. Jiang, J.-F. Liu and Q.-X. Zhou, Anal. Chim. Acta, 2003, 494, 149–156 CrossRef CAS.
- M. Abdel Salam and R. Burk, Anal. Bioanal. Chem., 2008, 390, 2159–2170 CrossRef CAS PubMed.
- B. Suárez, B. Santos, B. M. Simonet, S. Cárdenas and M. Valcárcel, J. Chromatogr. A, 2007, 1175, 127–132 CrossRef PubMed.
- R. Q. Long and R. T. Yang, J. Am. Chem. Soc., 2001, 123, 2058–2059 CrossRef CAS PubMed.
- M. A.-E. Abdallah, D. Drage and S. Harrad, Environ. Sci.: Processes Impacts, 2013, 15, 2279–2287 Search PubMed.
- J. O. Grimalt, B. L. van Drooge, A. Ribes, R. M. Vilanova, P. Fernandez and P. Appleby, Chemosphere, 2004, 54, 1549–1561 CrossRef CAS PubMed.
- S. Afful, J. Awudza, S. Twumasi and S. Osae, Chemosphere, 2013, 93, 1556–1560 CrossRef CAS PubMed.
- W. Ameur, S. Trabelsi, B. El Bedoui and M. Driss, Bull. Environ. Contam. Toxicol., 2011, 86, 539–544 CrossRef PubMed.
- A. Covaci, A. Gheorghe, S. Voorspoels, J. Maervoet, E. Steen Redeker, R. Blust and P. Schepens, Environ. Int., 2005, 31, 367–375 CrossRef CAS PubMed.
- R. Barra, P. Popp, R. Quiroz, C. Bauer, H. Cid and W. Tümpling, Chemosphere, 2005, 58, 905–915 CrossRef CAS PubMed.
- M. E. Aydin, S. Ozcan and A. Tor, Clean: Soil, Air, Water, 2007, 35, 660–668 CrossRef CAS.
- T. Brázová, V. Hanzelová and D. Miklisová, Parasitol. Res., 2012, 111, 779–786 CrossRef PubMed.
- UNEP, Stockholm convention on persistent organic pollutants (POPs), Secretariat of the Stockholm Convention on Persistent Organic Pollutants, Geneva, Switzerland, 2009 Search PubMed.
- S. Zeng, Y. Cao, W. Sang, T. Li, N. Gan and L. Zheng, Int. J. Mol. Sci., 2012, 13, 6382–6398 CrossRef CAS PubMed.
- X. Du, S. Lin, N. Gan, X. Chen, Y. Cao, T. Li and P. Zhan, J. Sep. Sci., 2014, 37, 1591–1600 CrossRef CAS PubMed.
- K. Gomez-Taylor, M. Kahn, H. D. Telliard, W. A. Ditthavong, K. Kopylev, L. McCarty, H. Riddick, L. Miller, K. Cuddeback, J. Rushneck, D. Dedah and S. Stralka, Technical support document for the assessment of detection and quantitation approaches, US Environmental Protection Agency, Washington, DC, 2003 Search PubMed.
- H.-H. Cho, B. A. Smith, J. D. Wnuk, D. H. Fairbrother and W. P. Ball, Environ. Sci. Technol., 2008, 42, 2899–2905 CrossRef CAS PubMed.
- K. Goh, L. Setiawan, L. Wei, W. Jiang, R. Wang and Y. Chen, J. Membr. Sci., 2013, 446, 244–254 CrossRef CAS.
- D. W. Seo, W. J. Yoon, S. J. Park, M. C. Jo and J. S. Kim, Carbon: Sci. Technol., 2006, 7, 266–270 Search PubMed.
- S. T. Kim, H. J. Choi and S. M. Hong, Colloid Polym. Sci., 2006, 285, 593–598 Search PubMed.
- H.-H. Cho, H. Huang and K. Schwab, Langmuir, 2011, 27, 12960–12967 CrossRef CAS PubMed.
- M. Abdel Salam, Arabian J. Chem., 2012, 5, 291–296 CrossRef CAS.
- W. Den, H. Liu, S. Chan, K. T. Kin and C. Huang, J. Environ. Eng. Manage., 2006, 16, 275–282 CAS.
- A. H. El-Sheikh, A. A. Insisi and J. A. Sweileh, J. Chromatogr. A, 2007, 1164, 25–32 CrossRef CAS PubMed.
- W. Chen, L. Duan and D. Zhu, Environ. Sci. Technol., 2007, 41, 8295–8300 CrossRef CAS PubMed.
- Y. W. Shiu and D. Mackay, J. Phys. Chem. Ref. Data, 1986, 15, 911–928 CrossRef.
- S. Dahane, M. D. Gil García, A. Uclés Moreno, M. Martínez Galera, M. D. M. Socías Viciana and A. Derdour, Microchim. Acta, 2015, 182, 95–103 CrossRef CAS.
- C. Sun, Y. Dong, S. Xu, S. Yao, J. Dai, S. Han and L. Wang, Environ. Pollut., 2002, 117, 9–14 CrossRef CAS PubMed.
- W.-L. Ho, Y.-Y. Liu and T.-C. Lin, Environ. Eng. Sci., 2011, 28, 421–434 CrossRef CAS.
- J. Lai, R. Niessner and D. Knopp, Anal. Chim. Acta, 2004, 522, 137–144 CrossRef CAS.
- M. Kragulj, J. Tričković, B. Dalmacija, Á. Kukovecz, Z. Kónya, J. Molnar and S. Rončević, Chem. Eng. J., 2013, 225, 144–152 CrossRef CAS.
- S. Gotovac, Y. Hattori, D. Noguchi, J. Miyamoto, M. Kanamaru, S. Utsumi, H. Kanoh and K. Kaneko, J. Phys. Chem. B, 2006, 110, 16219–16224 CrossRef CAS PubMed.
- F. Yu, J. Ma and Y. Wu, J. Hazard. Mater., 2011, 192, 1370–1379 CrossRef CAS PubMed.
- R. W. Coughlin and F. S. Ezra, Environ. Sci. Technol., 1968, 2, 291–297 CrossRef CAS.
- F. Yu, J. Ma and S. Han, Sci. Rep., 2014, 4, 1–8 Search PubMed.
- F. Ahnert, H. A. Arafat and N. G. Pinto, Adsorption, 2003, 9, 311–319 CrossRef CAS.
- X. Peng, Y. Li, Z. Luan, Z. Di, H. Wang, B. Tian and Z. Jia, Chem. Phys. Lett., 2003, 376, 154–158 CrossRef CAS.
- C. Lu, Y.-L. Chung and K.-F. Chang, Water Res., 2005, 39, 1183–1189 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |