Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Comparison of GC-MS, HPLC-MS and SIFT-MS in conjunction with multivariate classification for the diagnosis of Crohn's disease in urine†
M.
Cauchi
*a,
D. P.
Fowler
b,
C.
Walton
b,
C.
Turner
c,
R. H.
Waring
d,
D. B.
Ramsden
d,
J. O.
Hunter
e,
P.
Teale
f,
J. A.
Cole
d and
C.
Bessant
g
aSchool of Aerospace, Transport and Manufacturing (SATM), Cranfield University, Bedfordshire MK43 0AL, UK. E-mail: m.cauchi@cranfield.ac.uk
bSchool of Energy, Environment and Agrifood (SEEA), Cranfield University, Bedfordshire MK43 0AL, UK
cThe Department of Life, Health and Chemical Sciences, Open University, Milton Keynes, MK7 6AA, UK
dSchool of Biosciences, University of Birmingham, Birmingham B15 2TT, UK
eGastroenterology Research Unit, Addenbrooke's Hospital, Box 262, Cambridge CB2 0QQ, UK
fLGC Ltd, Newmarket Rd, Fordham, Cambridgeshire CB7 5WW, UK
gSchool of Biological and Chemical Sciences, Queen Mary University of London, Mile End Road, London E1 4NS, UK
First published on 1st September 2015
Abstract
The developed world has seen an alarming increase in the incidence of gastrointestinal diseases, among the most common of which is Crohn's disease (CD) in the young. The current “gold standard” techniques for diagnosis are often costly, time consuming, inefficient, invasive, and offer poor sensitivities and specificities. This paper compares the performances of three hyphenated instrumental techniques that have been suggested as rapid methods for the non-invasive diagnosis of CD from urine. These techniques are gas chromatography-mass spectrometry (GC-MS), high performance liquid chromatography-mass spectrometry (HPLC-MS) and selected ion flow tube mass spectrometry (SIFT-MS). Each of these techniques is followed by multivariate classification to provide a diagnosis based on the acquired data. The most promising results for potentially diagnosing CD was via HPLC-MS. An overall classification accuracy of 73% (74% specificity; 73% sensitivity) was achieved for differentiating CD from healthy controls, statistically significant at 95% confidence.
Introduction
The incidences of patients in the developed world being diagnosed with gastrointestinal diseases have been increasing in recent years. This may be attributable to a combination of life-style traits, particularly unhealthy diet involving foods high in saturated fat, starch and sugar, and lack of fruit and vegetables normally rich in anti-oxidants.1 This has led to an increased interest in research into the causes, prevention and possible cure of these diseases.2 Further to this are cases of food intolerance which could be due to abnormal fermentation processes occurring within the gut3 or a drastic change in diet leading to increased incidences of Crohn's disease.4Crohn's disease (CD) is a debilitating inflammatory bowel disease (IBD) causing inflammation of the mucosal lining in the gut.5–7 It is known that CD can affect any part of the gastrointestinal tract, whereas ulcerative colitis (UC) – another IBD – typically only affects the large colon. The presenting symptoms of CD and UC (chiefly abdominal pain and diarrhoea) are similar making differential diagnosis of these two conditions challenging. This is very important because the treatment required is different.8 There have been occasions in which a patient deemed to be suffering from UC is later diagnosed with CD. This is in conjunction with the discovery of new therapeutic agents employed to treat IBD.9,10
Colonoscopy and sigmoidoscopy are the current “gold standard” methods of diagnosing CD (and UC).11,12 Sigmoidoscopy permits a direct 5–20 minute examination of the lining of the rectum and the lower part of the colon by using a fibre-optic scope attached to a camera enabling the examiner to observe the lining for any irregularities. Colonoscopy makes use of a probe of greater length which is able to extend up to the ileum.13 The duration of the procedure can be 30 minutes in which it is necessary for the patient to be sedated. This tends to be a more accurate diagnostic technique than sigmoidoscopy.8,11 The two techniques are however highly invasive and expensive to perform.
An alternative approach is the determination of chemical biomarkers such as faecal calprotectin.14–17 However, the diagnostic performance of these tests is limited, which has led to the investigation of analytical approaches to the non-invasive diagnosis and monitoring of these conditions.18 More recently, analytical techniques incorporating mass spectrometric methods have been used to capture metabolic profiles of clinical samples. These can either analyse metabolites in solution19 or those in the vapour phase, so called volatile organic compounds (VOCs). These have advantages in that analysis can be non-invasive if urine, faeces, breath and some other fluids are analysed, reducing the need for invasive procedures which are uncomfortable and costly.
High performance liquid chromatography coupled with mass spectrometry (HPLC-MS) is routinely employed in proteomics,20 and gas chromatography mass spectrometry (GC-MS) techniques have been employed for many years for the detection of metabolites21 including the possible diagnosis of gastrointestinal diseases.22,23 HPLC-MS is also being employed in the study of metabolomics data such as the determination of the changes in the human urinary metabolome after the consumption of certain nuts, e.g. almonds,24,25 and the study of the age and strain-related differences in the Zucker rat.26,27 It has also been used in conjunction with proton nuclear magnetic resonance (1H NMR) for the analysis of biofluid samples.28–30
The relatively new technique of selected ion flow tube mass spectrometry (SIFT-MS) is also being employed in metabolomics.31–36 Rapid and quantitative analysis of VOCs can be achieved using SIFT-MS. This employs a fast flow tube to study the reaction of precursor ions with sample molecules in gas or vapour form. The flow tube technology, along with the quantitative mass spectrometry, allows selected precursor ions (H3O+, NO+, and O2+) to react in turn with the sample molecules to produce product ions through chemical ionisation (CI). These product ions are separated in a downstream quadrupole and are then detected and quantified. A kinetic database is used to quantify the concentrations of various molecules present in the sample. The particular precursor ions are chosen because they have slow reaction rates with the components of air, but react quickly with trace gases and vapours that may be used in research. This technology, unlike most CI techniques, is able to use all three reagents rapidly in turn on the same instrument.32 SIFT-MS is being widely employed for the real time analysis of volatile compounds originating from biological systems in medical applications33 and clinical diagnosis.34 A key advantage of SIFT-MS is the ability to distinguish between different isomers via the use of the three precursor ions mentioned previously.37
These analytical techniques all have the capability of producing large amounts of data about metabolites present, and therefore sophisticated techniques are needed in analysis. Multivariate classification is a pattern recognition technique which determines which samples belong to a designated class.21 One approach is partial least squares discriminant analysis (PLS-DA)38,39 and is termed a supervised method leading to the separation of samples into different classes, for example healthy and diseased. Although there exist more advanced techniques such as support vector machines40 and artificial neural networks,41 PLS-DA permits the direct identification of statistically significant features that may be related to potential biomarkers by visual inspection of the PLS loadings.39,42 A recent study reported the use of GC-MS in conjunction with PLS-DA for the diagnosis of gastrointestinal diseases including Crohn's disease in a series of matrices (faeces, breath, blood and urine).43 This study found that only CD could be diagnosed in the presence of the other diseases and healthy controls. This was achieved using faecal material. Very good accuracy was also attained for CD by analysing urine, but the sensitivity was below 50% and thus of no diagnostic benefit.
In looking at diagnostic or screening tests, the ease of sample acquisition and use is an important consideration. Faecal samples are generally harder to collect and process, not least of which is due to the subjects' reluctance to provide samples. Blood is also occasionally problematic due to the invasive nature of the sampling, and the discomfort caused to patients by venepuncture. For this reason, and the ease of collection and storage, urine is considered to be a better matrix to use, which is the reason why it was investigated in this study.
The present article describes the application of multivariate classification to GC-MS, HPLC-MS and SIFT-MS data acquired from the same urine samples which were employed in our previous paper.43 This was carried out in order to determine whether the greater sensitivity and overall accuracy was achieved compared with the results obtained using GC-MS data, the overall objective being to distinguish patients suffering from CD from those with IBS and UC and from healthy individuals.
Experimental
Reagents
Unless otherwise stated, analytical grade reagents and solvents were employed.Sampling
The study had been ethically approved by the National Research Ethics Service in Leeds in July 2007 (07/Q1205/39).
Instrumental measurements
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 resolution. The injection volume was 10 μl. A more detailed account of the instrumental parameters and conditions are listed in Table SM1 of the ESI.†
000 resolution. The injection volume was 10 μl. A more detailed account of the instrumental parameters and conditions are listed in Table SM1 of the ESI.†
The sample VOCs react with one of three precursor ions (H3O+, NO+ or O2+) to generate product ions, which are then separated via a quadrupole and counted (in counts per second) at the detector. Thus the data obtained are in the form of counts per second determined over a 30 second period at each mass to charge ratio (m/z), from m/z 10 to m/z 140. The data thus obtained represent the amount of product ion formed using each of the three precursor ions. Using this instrument, whole volatile profiles of samples may be generated very rapidly, offering real time instantaneous results as opposed to GC-MS and HPLC-MS which can only offer “snapshots” of instances in a particular time and space.
Data pre-processing
Correlation optimised warping (COW)47 was employed to align the chromatograms. This has the advantage of requiring minimum user input especially as the two main parameters (segment and slack) are determined automatically. A segment contains a fixed number of retention time ranges which contain peaks to be shifted. The extremity of the shifting is determined by the slack. A reference chromatogram must first be determined via a number of options such as the mean, median, maximum or the correlation coefficients. It is also possible to employ a PCA loading (typically PC1) as a reference chromatogram.48 The latter was employed here. After the segment and slack parameters were automatically determined, they were employed to align the respective chromatograms within the data matrix.
Principal components analysis (PCA) and correlation optimised warping (COW) was employed as for GC-MS.
Data analysis
The overall success of classification is determined in conjunction with the specificity and sensitivity. Specificity determines how well the healthy (control) samples were classified whilst sensitivity determines how well the target case (diseased) samples were classified.
Results and discussion
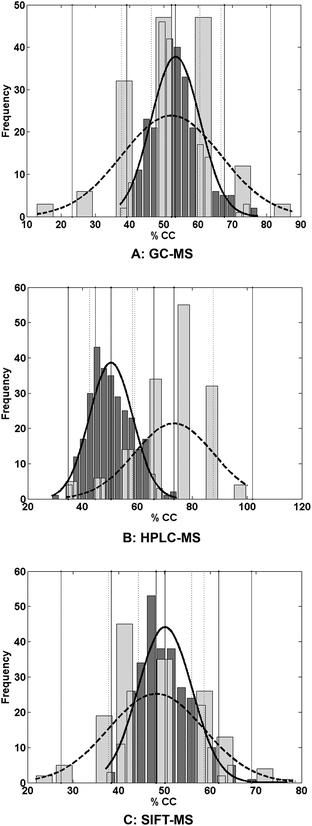
Table 1 summarises the results attained for the classification of Crohn's disease against the healthy control in the urine sample matrix for GC-MS, HPLC-MS and SIFT-MS.| Instrument | % CCa (%) | SPECa (%) | SENSa (%) | AUROCb | p value from z-test (α = 0.05) |
|---|---|---|---|---|---|
| a % CC: The overall correctly classified; SPEC: Specificity; SENS: Sensitivity. b Area under the receiver operating characteristic (AUROC) curve. | |||||
| GC-MS | 52.3 | 62.8 | 34.7 | 0.45 | 0.502 |
| HPLC-MS | 73.3 | 73.7 | 73.1 | 0.79 | <0.001 |
| SIFT-MS | 48.2 | 55.7 | 42.6 | 0.43 | 0.093 |
Fig. 1 displays the permutation test51 results comparing both the distribution of the permutations and the evaluations.
Table 1 and Fig. 1 show that, using urine as the analyte matrix, HPLC-MS in conjunction with multivariate classification is the best of the three methods for differentiating CD patients from healthy controls.
This is supported by the comparison of the means of the two distributions in which the calculated z-values were statistically significant for HPLC-MS but insignificant for both GC-MS and SIFT-MS.
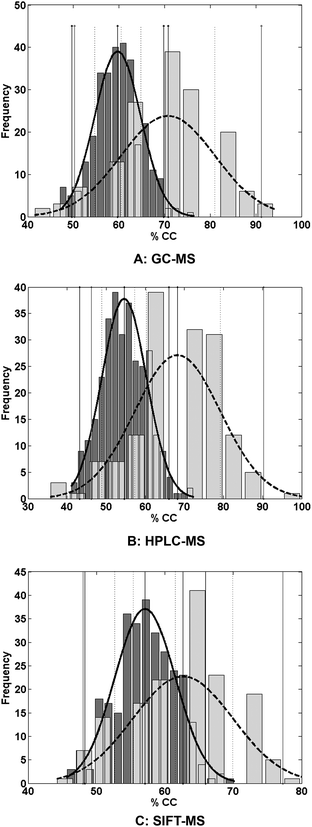
In clinical practice, the presenting symptoms are similar for a range of conditions. The data analysis was therefore repeated in order to differentiate CD in the presence of other diseases (IBS and UC) in addition to healthy controls. The results are summarized in Table 2. Fig. 2 shows the permutation test results for CD versus the healthy controls and other disease states comparing both the distribution of the permutations and the evaluations.
| Instrument | % CCa (%) | SPECa (%) | SENSa (%) | AUROCb | p value from z-test (α = 0.05) |
|---|---|---|---|---|---|
| a % CC: The overall correctly classified; SPEC: Specificity; SENS: Sensitivity. b Area under the receiver operating characteristic (AUROC) curve. | |||||
| GC-MS | 70.8 | 72.8 | 65.9 | 0.78 | <0.001 |
| HPLC-MS | 68.2 | 75.7 | 54.6 | 0.61 | <0.001 |
| SIFT-MS | 62.6 | 77.9 | 30.2 | 0.52 | <0.001 |
These results show that the discrimination of CD against healthy controls and other target cases is more challenging than separating CD from healthy controls only. This is evident in the sensitivities reported in Table 2 for HPLC-MS and SIFT-MS. By comparison, increased sensitivity was observed in the GC-MS data with overall classification accuracy (% CC) over 70% being attained. The specificities shown in Table 2 exceed 70%. This may be attributed to the imbalance of the respective datasets, resulting from combining the data from the other diseases (IBS and UC) with those of the healthy controls to form one “healthy” dataset. As a consequence the PLS-DA model is better trained to recognize the “healthy” class. However the sensitivity for GC-MS reported earlier in Table 1 was very low (∼35%), suggesting failure to distinguish the target case (CD) from the healthy controls. To investigate this phenomenon, the misclassification of IBS, UC and healthy controls as CD was investigated (Table 3).
| Case | Instrument | ||
|---|---|---|---|
| GC-MS | HPLC-MS | SIFT-MS | |
| CD | 66 | 55 | 30 |
| IBS | 26 | 25 | 22 |
| UC | 25 | 26 | 20 |
| CTRL | 30 | 23 | 25 |
Table 3 suggests that for GC-MS, 30% of control samples were misclassified as CD samples, whilst 26% and 25% of IBS and UC samples respectively were misclassified as CD. This explains why the sensitivity of ∼35% was attained for CD versus control only (Table 1), which was due to the difficulty in GC-MS distinguishing between controls and CD.
This is also apparent for SIFT-MS, because 25% of control samples were misclassified as CD, and only 30% of CD samples were correctly classified. In contrast, fewer control samples were misclassified as CD via HPLC-MS. Furthermore, 26% of UC samples were misclassified as CD, confirming the difficulty in distinguishing between CD and UC.9,10
Further interrogation of the loadings extracted from the PLS-DA model pertaining to the CD versus control dataset for HPLC-MS, resulted in a number of compounds being identified via the MassBank website (http://www.massbank.jp). These were moracin-C, 3-(3-hydroxyphenyl) propionic acid, chalcomoracin, dimethyl azelate, nonanedioic acid dimethyl ester and 9-hydroxyimino-6-methyl-4-oxo-6,7,8,9-tetrahydro-4H-pyrido(1,2-A) pyrimidine-3-carboxylic acid ethyl ester (“HMOTPPCAEE”). However, moracin-C and chalcomoracin were found to be antibacterial compounds and could be as a result of drugs taken by CD and IBS sufferers.56 Moracin-C was also found in IBS versus control. Of great interest, 3-(3-hydroxyphenyl) propionic acid, dimethyl azelate, nonanedioic acid dimethyl ester and “HMOTPPCAEE” could be potential biomarkers for CD (in urine via HPLC-MS) since no occurrences were identified in the control, IBS and UC samples.
Propionic acid had also been observed to be statistically significant in the faecal samples of patients presenting with CD57 which suggests that this could be a key metabolite since observed in both urine and faecal samples. There had also been increases in the concentrations of alcohol and ester derivatives of indole and some short-chain fatty acids such as 3-methyl butanoic acid in CD compared to UC and IBS.57
Furthermore, a key problem is in differentiating between Crohn's disease and ulcerative colitis. They present similarly but have different treatments. These metabolic profiling techniques should be used in conjunction with clinical symptoms. Other common conditions of the gastro-intestinal tract such as IBS may also be differentiated43,57 but generally have less severe symptoms. Moreover, many gastrointestinal diseases have similar symptoms such as pain, diarrhoea and weight loss, but very different pathology which may be reflected in separate biomarkers which are thus of potential diagnostic value. Lastly, a study in 2013 further illustrated that 25% of cases of CD do not get a diagnosis until two years have elapsed58 therefore highlighting the need for a more rapid diagnosis.
Conclusions
The comparison of the three instrumental techniques for the diagnosis of Crohn's disease (CD) using urine as the analyte matrix indicated that HPLC-MS was the best for distinguishing CD sufferers from healthy controls. Nevertheless when IBS and UC patients were included into the subject matrix together with healthy controls, GC-MS appeared to be the best method. However, the misclassification of the IBS, UC and healthy controls was taken into consideration (Table 3), it is possible that HPLC-MS is superior. SIFT-MS and GC-MS analyses were not sufficiently accurate, with unacceptably low sensitivities. These methods analyse VOCs, whereas HPLC-MS analyses metabolites in solution. The results obtained using HPLC-MS imply that the metabolites in solution are better indicators of CD than the volatile compounds present in urine headspace. Previous work has shown that VOCs in the headspace of faecal samples may be used in differentiating CD from UC and other IBDs,43,57 but use of urine headspace is less efficient as a means of classification.The typical accuracy of the “gold-standard method” of colonoscopy at the time of writing was 79%. Though the overall classification accuracies reported in this work did not exceed this value (e.g. 73% via HPLC-MS for CD vs. healthy control) it does suggest that urine could become a suitable matrix for the non-invasive diagnosis of CD. The gold standard for all gastrointestinal diseases remains endoscopy and the histological examination of tissue biopsies. Detection of specific biomarkers may help focus accurately the investigations required saving both time and expense.
This manuscript covered the potential for using this combination of analytical instrumentation with multivariate statistics for disease diagnosis. Further work would concentrate on validating this technology, and then the diagnostic potential would be in rolling this approach out in clinics, where it is often difficult to diagnose Crohn's disease except via endoscopy or sigmoidoscopy.
Acknowledgements
We gratefully acknowledge the Wellcome Trust for funding the work (project 080238/Z/06/Z).We also gratefully acknowledge the following persons for their valuable contribution to the work: Bruce Bolt, Philip Spratt, Wenjing Jia, Hao Bai, Claire Dawson, Lesley Griffiths and Rebekah Whitehead.
References
- S. Bengmark, Clin. Nutr., 2004, 23, 1256–1266 CrossRef PubMed.
- J. G. Williams, S. E. Roberts, M. F. Ali, W. Y. Cheung, D. R. Cohen, G. Demery, A. Edwards, M. Greer, M. D. Hellier, H. A. Hutchings, B. Ip, M. F. Longo, I. T. Russell, H. A. Snooks and J. C. Williams, Gut, 2007, 56, 1–113 CrossRef PubMed.
- J. O. Hunter, Lancet, 1991, 338, 495–496 CrossRef CAS.
- R. Shoda, K. Matsueda, S. Yamato and N. Umeda, Am. J. Clin. Nutr., 1996, 63, 741–745 CAS.
- R. M. Nakamura, M. Matsutani and M. Barry, Clin. Chim. Acta, 2003, 335, 9–20 CrossRef CAS.
- P. von Stein, N. Kouznetsov, A. Gielen, Ä. Öst, O. von Stein and R. Lofberg, Journal of Crohn's and Colitis Supplements, 2007, 1, 12 CrossRef.
- P. von Stein, R. Lofberg, N. V. Kuznetsov, A. W. Gielen, J.-O. Persson, R. Sundberg, K. Hellstrom, A. Eriksson, R. Befrits, A. Ost and O. D. von Stein, Gastroenterology, 2008, 134, 1869–1881 CrossRef PubMed.
- D. E. Deutsch and A. D. Olson, J. Pediatr. Gastroenterol. Nutr., 1997, 25, 26–31 CrossRef CAS.
- B. Moum, A. Ekbom, M. H. Vatn, E. Aadland, J. Sauar, I. Lygren, T. Schulz, N. Stray and O. Fausa, Gut, 1997, 40, 328–332 CrossRef CAS.
- E. Seidman and C. Deslandres, Pitfalls in the diagnosis and management of pediatric IBD, Kluwer Academic Publishing, Lancaster, 1997 Search PubMed.
- D. S. Fefferman and R. J. Farrell, Clin. Gastroenterol. Hepatol., 2005, 3, 11–24 CrossRef.
- G. Manes, V. Imbesi, S. Ardizzone, A. Cassinotti, M. Bosani, A. Massari and G. Bianchi Porro, Dig. Liver Dis., 2009, 41, 653–658 CrossRef CAS PubMed.
- Anonymous, health-cares.net, 2005.
- I. Angriman, M. Scarpa, R. D'Incà, D. Basso, C. Ruffolo, L. Polese, G. C. Sturniolo, D. F. D'Amico and M. Plebani, Clin. Chim. Acta, 2007, 381, 63–68 CrossRef CAS PubMed.
- J. L. Mendoza and M. T. Abreu, Gastroenterol. Clin. Biol., 2009, 33, S158–S173 CrossRef CAS.
- M. Moscandrew and E. Loftus, Curr. Gastroenterol. Rep., 2009, 11, 488–495 CrossRef.
- P. F. van Rheenen, E. van de Vijver and V. Fidler, Br. Med. J., 2010, 341, c3369 CrossRef PubMed.
- C. Turkay and B. Kasapoglu, Clinics, 2010, 65, 221–231 CrossRef PubMed.
- J. F. Xiao, B. Zhou and H. W. Ressom, TrAC, Trends Anal. Chem., 2012, 32, 1–14 CrossRef CAS PubMed.
- P. Alex, M. Gucek and X. Li, Inflammatory Bowel Dis., 2009, 15, 616–629 CrossRef PubMed.
- K. K. Pasikanti, P. C. Ho and E. C. Y. Chan, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2008, 871, 202–211 CrossRef CAS PubMed.
- C. E. Garner, S. Smith, P. K. Bardhan, N. M. Ratcliffe and C. S. J. Probert, Trans. R. Soc. Trop. Med. Hyg., 2009, 103, 1171–1173 CrossRef CAS PubMed.
- C. S. J. Probert, I. Ahmed, T. Khalid, E. Johnson, S. Smith and N. Ratcliffe, J. Gastrointestin. Liver Dis., 2009, 18, 337–344 Search PubMed.
- R. Llorach, I. Garrido, M. a. Monagas, M. Urpi-Sarda, S. Tulipani, B. Bartolome and C. Andres-Lacueva, J. Proteome Res., 2010, 9, 5859–5867 CrossRef CAS PubMed.
- S. Tulipani, R. Llorach, O. Jáuregui, P. López-Uriarte, M. Garcia-Aloy, M. Bullo, J. Salas-Salvadó and C. Andrés-Lacueva, J. Proteome Res., 2011, 10, 5047–5058 CrossRef CAS PubMed.
- J. H. Granger, R. Williams, E. M. Lenz, R. S. Plumb, C. L. Stumpf and I. D. Wilson, Rapid Commun. Mass Spectrom., 2007, 21, 2039–2045 CrossRef CAS PubMed.
- R. E. Williams, E. M. Lenz, J. A. Evans, I. D. Wilson, J. H. Granger, R. S. Plumb and C. L. Stumpf, J. Pharm. Biomed. Anal., 2005, 38, 465–471 CrossRef CAS PubMed.
- J. M. Garcia-Manteiga, S. Mari, M. Godejohann, M. Spraul, C. Napoli, S. Cenci, G. Musco and R. Sitia, J. Proteome Res., 2011, 10, 4165–4176 CrossRef CAS PubMed.
- R. E. Williams, E. M. Lenz, J. S. Lowden, M. Rantalainen and I. D. Wilson, Mol. BioSyst., 2005, 1, 166–175 RSC.
- I. D. Wilson, R. Plumb, J. Granger, H. Major, R. Williams and E. M. Lenz, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2005, 817, 67–76 CrossRef CAS PubMed.
- N. G. Adams and D. Smith, Int. J. Mass Spectrom. Ion Phys., 1976, 21, 349–359 CrossRef CAS.
- P. Španěl and D. Smith, Med. Biol. Eng. Comput., 1996, 34, 409–419 Search PubMed.
- P. Španěl and D. Smith, Eur. J. Mass Spectrom., 2007, 13, 77–82 CrossRef PubMed.
- D. Smith and P. Španěl, Mass Spectrom. Rev., 2005, 24, 661–700 CrossRef CAS PubMed.
- P. Španěl and D. Smith, Mass Spectrom. Rev., 2011, 30, 236–267 CrossRef PubMed.
- K. Hopes, M. Cauchi, C. Walton, H. MacQueen, W. Wassif and C. Turner, Analyst, 2015, 140, 3028–3038 RSC.
- D. Smith, K. Sovová and P. Španěl, Int. J. Mass Spectrom., 2012, 319–320, 25–30 CrossRef CAS PubMed.
- M. Barker and W. Rayens, J. Chemom., 2003, 17, 166–173 CrossRef CAS PubMed.
- S. Wiklund, E. Johansson, L. Sjostrom, E. J. Mellerowicz, U. Edlund, J. P. Shockcor, J. Gottfries, T. Moritz and J. Trygg, Anal. Chem., 2008, 80, 115–122 CrossRef CAS PubMed.
- M. Sattlecker, C. Bessant, J. Smith and N. Stone, Analyst, 2010, 135, 895–901 RSC.
- M. T. Hagan, H. B. Demuth and M. Beale, Neural Network Design, International Thompson Publishing, Boston, 1996 Search PubMed.
- J. Trygg, E. Holmes and T. R. Lundstedt, J. Proteome Res., 2007, 6, 469–479 CrossRef CAS PubMed.
- M. Cauchi, C. Walton, D. Fowler, C. Turner, W. Jia, W. Rebekah, L. Griffiths, C. Dawson, R. Waring, D. B. Ramsden, J. A. Cole, C. Bessant and J. O. Hunter, Metabolomics, 2014, 10, 1113–1120 CrossRef CAS.
- S. Wold, K. Esbensen and P. Geladi, Chemom. Intell. Lab. Syst., 1987, 2, 37–52 CrossRef CAS.
- H. Hotelling, Ann. Math. Stat., 1931, 2, 360–378 CrossRef.
- M. Otto, Chemometrics: Statistics and Computer Applications in Analytical Chemistry, Wiley-VCH, Germany, 1999 Search PubMed.
- G. Tomasi, F. van den Berg and C. Andersson, J. Chemom., 2004, 18, 231–241 CrossRef CAS PubMed.
- T. Skov, F. van den Berg, G. Tomasi and R. Bro, J. Chemom., 2006, 20, 484–497 CrossRef CAS PubMed.
- N. Takahashi, G. Boysen, F. Li, Y. Li and J. A. Swenberg, Kidney Int., 2007, 71, 266–271 CrossRef CAS PubMed.
- S. de Jong, Chemom. Intell. Lab. Syst., 1993, 18, 251–263 CrossRef CAS.
- R. G. Brereton, Chemometrics for Pattern Recognition, John Wiley & Sons, Chichester, UK, 2009 Search PubMed.
- Q. Rahman and G. Schmeisser, Numer. Math., 1990, 57, 123–138 CrossRef.
- M. H. Zweig and G. Campbell, Clin. Chem., 1993, 39, 561–577 CAS.
- J. Westerhuis, H. Hoefsloot, S. Smit, D. Vis, A. Smilde, E. van Velzen, J. van Duijnhoven and F. van Dorsten, Metabolomics, 2008, 4, 81–89 CrossRef CAS.
- M. J. Campbell and D. Machin, Medical Statistics: A Common Sense Approach, 3rd edn, John Wiley & Sons Ltd., Chichester, UK, 1999 Search PubMed.
- Y. J. Kim, M.-J. Sohn and W.-G. Kim, Biol. Pharm. Bull., 2012, 35, 791–795 CAS.
- C. Walton, D. P. Fowler, C. Turner, W. Jia, R. N. Whitehead, L. Griffiths, C. Dawson, R. H. Waring, D. B. Ramsden, J. A. Cole, M. Cauchi, C. Bessant and J. O. Hunter, Inflammatory Bowel Dis., 2013, 19, 2069–2078 CrossRef PubMed.
- A. M. Schoepfer, M.-A. Dehlavi, N. Fournier, E. Safroneeva, A. Straumann, V. Pittet, L. Peyrin-Biroulet, P. Michetti, G. Rogler and S. R. Vavricka, Am. J. Gastroenterol., 2013, 108, 1744–1753 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ay01322d |
| This journal is © The Royal Society of Chemistry 2015 |