Open Access Article
Open Access ArticleAn on-line solid phase extraction method coupled with UHPLC-MS/MS for the determination of steroid hormone compounds in treated water samples from waste water treatment plants
Rayco
Guedes-Alonso
,
Zoraida
Sosa-Ferrera
and
José Juan
Santana-Rodríguez
*
Departamento de Química, Universidad de Las Palmas de Gran Canaria, 35017, Las Palmas de Gran Canaria, Spain. E-mail: josejuan.santana@ulpgc.es
First published on 9th June 2015
Abstract
An on-line solid phase extraction coupled with ultra-high performance liquid chromatography in tandem with mass spectrometry (SPE-UHPLC-MS/MS) method for the determination of fourteen hormones (four oestrogens, three androgens, four progestogens and three corticosteroids) in waste water samples has been developed. All of the parameters involved in the on-line extraction process have been optimized: type of cartridge, sample volume, loading solvent, solvent of the wash step and the pH of the sample. Moreover, the chromatographic separation and all of the parameters involved in the detection by mass spectrometry have been studied too. The developed method allows for complete analysis (extraction and identification of the analytes) in 14.5 minutes. The method is selective, with satisfactory relative standard deviations (lower than 15% in most cases) and limits of detection and quantification that ranged from 0.5 to 13.2 ng L−1 and from 1.66 to 44 ng L−1, respectively. The recoveries were acceptable for most compounds for effluent samples from different waste water treatment plants (between 50 and 90%). The proposed method has been applied to study effluent samples from three waste water treatment plants from Gran Canaria (Spain). Four steroid hormones of different families have been detected at concentrations ranging from 3.1 to 52.8 ng L−1.
1. Introduction
In recent years, endocrine disrupting compounds (EDCs) have garnered increasing attention from the international community. Changes in aquatic biota, such as hermaphroditism, feminization, inhibition of locomotion and aggressive behaviour or changes in fertility or vitellogenin, are produced by these types of emerging pollutants, which have been discussed in several studies.1–4 Among the EDCs, we can consider steroid hormones as a wide group that can be divided into four subgroups: oestrogens, androgens, progestogens and corticosteroids. Oestrogens, such as 17β-estradiol (E2), estrone (E1) and oestriol (E3), are female hormones that are essential to the menstrual cycle of women. Natural and synthetic oestrogens are used in both human and veterinary medicine with the main medical application being birth control. The most used synthetic oestrogen for birth control is 17α-ethinyloestradiol (EE). Progestogens, also called gestagens, are characterized by their basic 21-carbon skeleton and their main function is to maintain the pregnancy, although they are expressed in several phases of the menstrual cycle. Consequently, progestogens are also used as hormonal contraceptives that can be combined with oestrogens. In the last decade, the consumption of oestrogens, with and without combination with progestogens, has greatly increased. In fact, currently, 100 million women are active users of combined hormonal contraceptives worldwide.5 Alternatively, androgens are frequently used by some sportsmen to increase their strength, mass and muscular size. Nevertheless, the doses are higher than the doses used in hormone replacement therapies; in consequence, serious side effects can appear, such as testicular atrophy, sterility, gynecomastia in males and ovulation inhibition, hirsutism, alopecia and acne in females.6 Finally, corticosteroids are synthesized in the adrenal cortex of vertebrates and are involved in many physiological processes. Corticosteroids are divided into mineralocorticoids and glucocorticoids and these substances can be artificially synthesized for therapeutic applications due to their anti-inflammatory properties and immunosuppressive effects on metabolism. Corticosteroids are illegal in the EU for fattening purposes as legislated in the 96/22/EC directive.7A significant quantity of consumed hormones exit organisms through excretions.8,9 For this reason, most publications agree that waste water treatment plants (WWTPs) are the principal sources of EDC release into the environment.10 The presence of hormone compounds in the effluents of WWTPs is due to their incomplete degradation by treatment processes, which produces an alarming contamination in aquatic environments.11–13 The compound concentrations found in the environment are in the range of ng L−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12,14,15 because of the low doses of these drugs, their catabolism by humans and most of them degrade in WWTPs.
12,14,15 because of the low doses of these drugs, their catabolism by humans and most of them degrade in WWTPs.
Because of the low concentration of steroid hormones in the environment, it is necessary to develop sensitive methods for extraction, preconcentration and identification of hormones in water samples. Solid phase extraction (SPE) is a widespread method of extraction used to isolate and preconcentrate emerging pollutants from aqueous matrices.16–18 Some authors have reported extraction of oestrogens, androgens, progestogens and corticosteroids from WWTP samples using this method in the last decade.18–21 The separation and identification techniques used more often in recent years have been liquid chromatography with mass spectrometry detection (LC-MS)22 and liquid chromatography in tandem with mass spectrometry detection (LC-MS/MS).19,20,23,24 These techniques allow the identification of hormones without a derivatization step, which is needed when using GC-MS.25–27 On-line SPE methods have been developed in recent years and present advantages over off-line SPE methods, such as lower sample handling and analysis time. On-line SPE coupled to HPLC-MS/MS and UHPLC-MS/MS provides a highly sensitive and specific method for steroid hormone detection in water samples.20,24,28
In this study, an on-line SPE process coupled with liquid chromatography in tandem with mass spectrometry detection system has been developed for the determination of fourteen steroid hormones belonging to four subgroups (Table 1). All of the conditions involved in the extraction, separation and identification processes have been optimized using the effluent from a tertiary treatment used at a waste water treatment plant (WWTP1). The developed method has been applied to study effluent samples from three WWTPs (WWTP2, WWTP3 and WWTP4) located in Gran Canaria Island (Spain) which use different water treatments. WWTP2 uses a membrane bioreactor for biological treatment and WWTP3 and WWTP4 use the traditional activated sludge treatment.
| Type of hormone | Abbreviation | Compound | Surrogate standard | pKa35 | t R (min) |
|---|---|---|---|---|---|
| Oestrogens | E3 | Estriol | Estrone D2 | 10.3 | 6.50 |
| E2 | 17β-estradiol | 10.3 | 7.07 | ||
| E1 | Estrone | 10.3 | 7.07 | ||
| DES | Diethylstilbestrol | 10.2 | 7.08 | ||
| Progestogens | NORET | Norethisterone | Progesterone D9 | 13.1 | 7.05 |
| NOR | Norgestrel | 13.1 | 7.20 | ||
| MGA | Megestrol acetate | — | 7.32 | ||
| PRO | Progesterone | — | 7.40 | ||
| Androgens | BOL | Boldenone | Testosterone D3 | 15.1 | 7.00 |
| NAN | Nandrolone | 15.1 | 7.05 | ||
| TES | Testosterone | 15.1 | 7.15 | ||
| Corticosteroids | PRED | Prednisone | Progesterone D9 | 12.4 | 6.60 |
| COR | Cortisone | 12.4 | 6.63 | ||
| PREDNL | Prednisolone | 12.5 | 6.73 |
2. Materials and methods
2.1. Reagents
All of the hormone compounds were purchased from Sigma-Aldrich (Madrid, Spain). The purities of all compounds under study are over 99.0%. Three surrogate standards were used: estrone D2 and progesterone D9 from CDN Isotopes Inc. (Quebec, Canada) and testosterone D3 from Toronto Research Chemicals Inc. (Toronto, ON, Canada). Stock solutions containing 1000 mg L−1 of each analyte were prepared by dissolving the compound in methanol and stored in glass-stoppered bottles at −20 °C prior to use. Working aqueous standard solutions were prepared daily. Ultrapure water used in the on-line SPE process was obtained by using a Milli-Q system (Millipore, Bedford, MA, USA). HPLC-grade methanol, LC-MS methanol, LC-MS water, HPLC-grade n-hexane and HPLC-grade acetone, as well as ammonia, ammonium acetate and acetic acid used to adjust the pH of the mobile phases, were obtained from Panreac Química (Barcelona, Spain).2.2. Sample collection
Water samples were collected from the effluents of four waste water treatment plants located in Gran Canaria in May of 2014 and January of 2015. WWTP1 samples were from the effluent of the tertiary process and were used to optimize the on-line SPE method. WWTP2 has a population equivalent of 7000 and uses a membrane bioreactor treatment system. WWTP3 and WWTP4 treat the water from big urban areas of Gran Canaria (population equivalents of 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 130
000 and 130![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, respectively) and use an activated sludge treatment. The samples were collected in 2 L amber glass bottles that were rinsed beforehand with methanol and ultrapure water. Samples were acidified to inhibit microbial activity and purified through filtration with fibreglass filters and then with 0.22 μm membrane filters (Millipore, Ireland). The samples were stored in the dark at 4 °C and they were analyzed within 48 hours.
000, respectively) and use an activated sludge treatment. The samples were collected in 2 L amber glass bottles that were rinsed beforehand with methanol and ultrapure water. Samples were acidified to inhibit microbial activity and purified through filtration with fibreglass filters and then with 0.22 μm membrane filters (Millipore, Ireland). The samples were stored in the dark at 4 °C and they were analyzed within 48 hours.
2.3. On-line SPE-UHPLC system
The on-line SPE-UHPLC-MS/MS system used was obtained from Waters (Waters Chromatography, Barcelona, Spain). This system consisted of a binary solvent manager (BSM) pump for the chromatographic separation, a quaternary solvent manager (QSM) pump to perform the extraction process, an autosampler capable of injecting volumes up to 5000 μL per injection, a column manager and a triple quadrupole detector (TQD). Solid phase extraction was performed using OASIS HLB (2.1 × 30 mm, 20 μm) and XBridge C18 (2.1 × 30 mm, 10 μm) SPE columns (Waters Chromatography, Barcelona, Spain) followed by elution of the analytes with the chromatographic mobile phase and finally, separation was achieved in the analytical column placed in the column manager.A scheme of the on-line SPE process is shown in Fig. 1. First, the autosampler injects a volume of up to 5000 μL into valve 2 and the sample is placed in the loop (Fig. 1a). Next is the loading phase (solvent A of the QSM) where the sample is loaded into the SPE column 1. After loading, solvents B and C of the QSM perform the wash step to eliminate interferents in the sample (Fig. 1b). After the wash step, a change in the valves allows for column 2 to be strongly washed with a mixture of organic solvents (solvent D of the QSM) while SPE column 1 is eluted with the chromatographic mobile phase of the binary solvent manager (BSM) (Fig. 1c). After the strong wash and during the chromatographic separation, SPE column 2 is conditioned and equilibrated with the load phase (solvent A of the QSM) to prepare it for the next extraction.
In this system the solvent pumps have different purposes. The quaternary solvent manager (QSM) is used to load the sample into the SPE column, a weak wash of the SPE column to eliminate interferents and to strongly wash the SPE columns to eliminate any analyte retention. The binary solvent manager serves for elution of the analytes to the separation column and chromatographic analyses.
2.4. Chromatographic and mass spectrometry conditions
| Time (min) | Binary solvent manager | Quaternary solvent manager | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Flow (mL min−1) | A (%) | B (%) | Flow (mL min−1) | A2 (%) | B2 (%) | C (%) | D (%) | ||
| OASIS HLB | XBridge C18 | ||||||||
a A: water + 0.1% NH3, A2: water + 0.05% formic acid, B: methanol, B2: water, C: methanol, D: acetone![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol (1 methanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1).
|
|||||||||
| 0.0 | 0.30 | 80 | 20 | 2.00 | 2.00 | 100 | 0 | 0 | 0 |
| 3.8 | 0.30 | 80 | 20 | 2.00 | 0.01 | 0 | 100 | 0 | 0 |
| 4.1 | 0.30 | 80 | 20 | 2.00 | 0.01 | 0 | 100 | 0 | 0 |
| 7.0 | 0.30 | 0 | 100 | 2.00 | 2.00 | 0 | 0 | 0 | 100 |
| 8.0 | 0.30 | 0 | 100 | 2.00 | 2.00 | 100 | 0 | 0 | 0 |
| 10.5 | 0.30 | 80 | 20 | 2.00 | 2.00 | 100 | 0 | 0 | 0 |
The detailed MS/MS detection parameters for each hormone compound are presented in Table 3 and the multiple reaction monitoring (MRM) parameters were optimised by the direct injection of a 1 mg L−1 standard solution of each analyte into the detector at a flow rate of 10 μL min−1.
| Compound | Precursor ion (m/z) | Cone voltage (ion mode) | Quantification ion, m/z (collision potential, V) | Confirmation ion, m/z, (collision potential, V) | Confirmation – Quantification ion ratio |
|---|---|---|---|---|---|
| E3 | 287.2 | −65 V (ESI−) | 171.0 (37) | 145.2 (39) | 0.19 |
| PRED | 359.3 | 30 V (ESI+) | 147.0 (15) | 237.0 (20) | 0.25 |
| COR | 361.3 | 30 V (ESI+) | 163.0 (25) | 121.0 (45) | 0.10 |
| PREDNL | 361.3 | 20 V (ESI+) | 147.1 (20) | 173.1 (25) | 0.39 |
| BOL | 287.2 | 30 V (ESI+) | 121.0 (28) | 135.1 (15) | 0.59 |
| NAN | 275.2 | 35 V (ESI+) | 109.1 (20) | 83.0 (30) | 0.53 |
| NORET | 299.2 | 30 V (ESI+) | 109.1 (25) | 91.0 (40) | 0.59 |
| E2 | 271.2 | −65 V (ESI−) | 145.1 (40) | 183.1 (31) | 0.23 |
| E1 | 269.2 | −65 V (ESI−) | 145.0 (36) | 143.0 (48) | 0.22 |
| DES | 267.1 | −50 V (ESI−) | 237.1 (29) | 251.1 (25) | 0.91 |
| TES | 289.2 | 38 V (ESI+) | 97.0 (22) | 109.0 (21) | 0.80 |
| NOR | 313.2 | 38 V (ESI+) | 109.0 (26) | 245.1 (18) | 0.56 |
| MGA | 385.5 | 30 V (ESI+) | 267.3 (15) | 224.2 (30) | 0.66 |
| PRO | 315.3 | 30 V (ESI+) | 97.0 (18) | 109.1 (25) | 0.86 |
| Deuterated Compound | Precursor ion (m/z) | Cone voltage (ion mode) | Quantification ion, m/z (collision potential, V) | Confirmation ion, m/z (collision potential, V) | Confirmation – quantification ion ratio |
|---|---|---|---|---|---|
| E1-d2 | 271.2 | 70 V (ESI−) | 147.1 (30) | 145.1 (35) | 0.12 |
| TES-d3 | 292.2 | 35 V (ESI+) | 97.1 (25) | 109.1 (20) | 0.80 |
| PRO-d9 | 324.3 | 35 V (ESI+) | 100.1 (20) | 113.1 (20) | 0.56 |
3. Results and discussion
3.1. Optimization of solid-phase extraction (SPE)
Another important parameter is the composition of the sample loading solvent because this solvent could improve or diminish the adsorption of analytes onto the SPE column and eliminate interferents from the matrix evaluated. The sample loading solvent is Milli-Q water and four conditions have been evaluated: with 0.1% (v/v) of NH3 (pH = 10.1), with 0.03% (v/v) of NH3 and 100 mM of ammonium acetate (pH = 8.1), with 0.05% (v/v) of acetic acid (pH = 3.4) and without additives (pH = 5.6).
For both SPE columns tested, maximum recoveries of most compounds were found when the sample volume is between 2 and 3 mL while volumes of 4 and 5 mL showed a significant decrease in recoveries, which may be due to the same sample producing a partial elution of the analytes. For the load phase, the pH between 3.4 and 5.6 showed better recoveries for OASIS HLB SPE columns while, XBridge C18 showed maximum recoveries at pH = 10.4.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) organic solvents (100
organic solvents (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, 90
0, 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 80
10, 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, 70
20, 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 and 60
30 and 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40) specifically methanol and water, with and without 0.1% of NH3. Percentages greater than 40% of methanol have not been tested to avoid a co-elution of the analytes under study.
40) specifically methanol and water, with and without 0.1% of NH3. Percentages greater than 40% of methanol have not been tested to avoid a co-elution of the analytes under study.
For XBridge C18 SPE columns, the wash step was eliminated because it caused the elution of the analytes retained in the column. For this reason, the flow rate of the wash step was reduced to the minimum that the UHPLC-MS/MS system allows for (0.01 mL min−1).
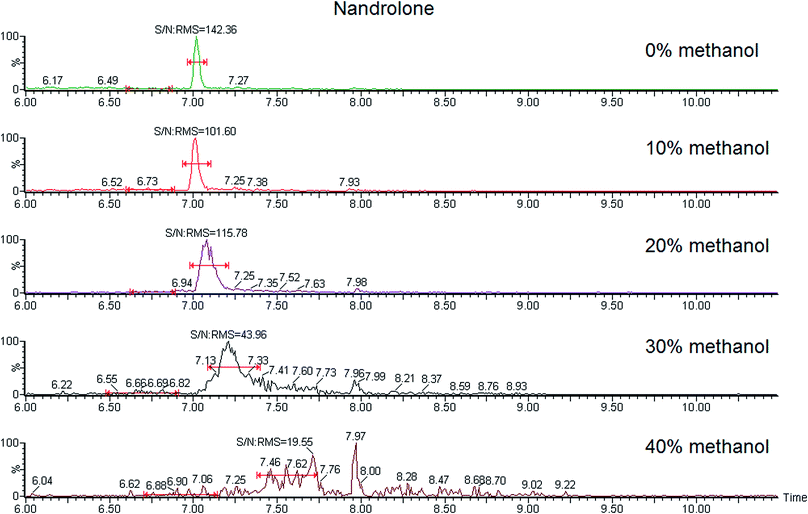
For OASIS HLB SPE columns the use of a wash step without NH3 produced higher recoveries and better S/N ratios for most compounds than the wash step with NH3. An acid wash step has not been tested because it produced the elution of the analytes. Regarding the mixture composition of the wash step, the best results were obtained without adding methanol because the presence of an organic solvent results in deformation of the peaks for most compounds. Fig. 2 shows the peak of nandrolone as an example of this deformation and loss of the S/N ratio at different proportions of aqueous![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) organic solvents used in the wash step.
organic solvents used in the wash step.
Once all the parameters were optimised, we selected the OASIS HLB SPE column, because with this SPE column, the recoveries were higher (over 60% for most compounds) than the recoveries obtained with XBridge C18 columns (between 9 and 57%).
3.2. Analytical parameters and quality control
Linearity, recovery, repeatability, limits of detection and limits of quantification were evaluated for each waste water sample (Table 4) using OASIS HLB SPE columns under the optimum extraction conditions. External calibration curves were prepared from 0.5 to 400 μg L−1 of each compound. Moreover, three surrogate standards (estrone D2, testosterone D3 and progesterone D9), at a fixed concentration of 200 μg L−1, were added to each calibration level. The linearity was calculated using the relationship between areas and concentrations of compounds and surrogates with excellent correlation coefficients (r2) higher than 0.990.| Compound | LODa (ng L−1) | Effluent WWTP1 | Effluent WWTP2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 100 ng L−1 | 500 ng L−1 | 100 ng L−1 | 500 ng L−1 | ||||||
| Recovery (%) n = 6 | RSDb (%) n = 6 | Recovery (%) n = 6 | RSDb (%) n = 6 | Recovery (%) n = 6 | RSDb (%) n = 6 | Recovery (%) n = 6 | RSDb (%) n = 6 | ||
| a Limit of detection. b Relative standard deviation. | |||||||||
| Diethylstilbestrol | 13.2 | 44.3 | 7.3 | 42.3 | 14.7 | 44.3 | 14.6 | 51.9 | 4.7 |
| 17β-estradiol | 8.5 | 88.8 | 26.4 | 104.0 | 7.0 | 126.7 | 14.6 | 112.8 | 6.2 |
| Estrone | 4.1 | 75.1 | 15.1 | 81.6 | 8.8 | 75.5 | 15.8 | 82.6 | 5.3 |
| Estriol | 4.5 | 76.8 | 5.2 | 69.7 | 17.1 | 58.6 | 16.9 | 78.5 | 11.0 |
| Norgestrel | 1.6 | 34.5 | 8.6 | 36.7 | 11.6 | 42.5 | 8.1 | 48.4 | 6.1 |
| Testosterone | 1.0 | 53.1 | 6.9 | 52.3 | 3.7 | 69.7 | 6.3 | 74.4 | 2.8 |
| Megestrol acet. | 1.2 | 138.7 | 6.8 | 154.4 | 10.8 | 153.6 | 11.4 | 195.9 | 3.7 |
| Prednisone | 9.2 | 61.7 | 11.5 | 60.7 | 5.0 | 97.5 | 9.8 | 82.3 | 12.0 |
| Prednisolone | 6.1 | 95.2 | 9.4 | 100.0 | 8.7 | 133.0 | 7.3 | 120.4 | 4.8 |
| Cortisone | 2.1 | 69.5 | 7.3 | 66.3 | 3.2 | 88.7 | 13.1 | 86.9 | 6.0 |
| Boldenone | 0.7 | 61.1 | 4.5 | 67.5 | 2.7 | 95.7 | 6.3 | 106.9 | 2.1 |
| Norethisterone | 2.3 | 42.7 | 2.9 | 44.3 | 3.3 | 73.3 | 9.5 | 76.9 | 2.5 |
| Nandrolone | 4.1 | 59.0 | 9.6 | 59.6 | 3.3 | 87.6 | 6.1 | 88.3 | 3.3 |
| Progesterone | 0.5 | 63.4 | 10.7 | 61.7 | 10.3 | 59.8 | 5.8 | 70.5 | 4.3 |
| Compound | LODa (ng L−1) | Effluent WWTP3 | Effluent WWTP4 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 100 ng L−1 | 500 ng L−1 | 100 ng L−1 | 500 ng L−1 | ||||||
| Recovery (%) n = 6 | RSDb (%) n = 6 | Recovery (%) n = 6 | RSDb (%) n = 6 | Recovery (%) n = 6 | RSDb (%) n = 6 | Recovery (%) n = 6 | RSDb (%) n = 6 | ||
| Diethylstilbestrol | 13.2 | 60.0 | 15.4 | 58.2 | 15.0 | 52.4 | 18.8 | 53.2 | 6.3 |
| 17β-estradiol | 8.5 | — | — | — | — | — | — | — | — |
| Estrone | 4.1 | 104.2 | 11.4 | 121.2 | 3.7 | 94.7 | 10.2 | 88.9 | 7.1 |
| Estriol | 4.5 | 54.4 | 10.1 | 59.1 | 15.1 | 114.1 | 11.4 | 89.4 | 15.7 |
| Norgestrel | 1.6 | 53.9 | 10.2 | 48.5 | 3.8 | 36.1 | 13.8 | 49.1 | 8.1 |
| Testosterone | 1.0 | 30.2 | 8.7 | 47.6 | 3.3 | 46.6 | 12.2 | 60.9 | 7.1 |
| Megestrol acet. | 1.2 | 43.8 | 5.5 | 58.9 | 3.9 | 19.3 | 14.9 | 40.3 | 14.7 |
| Prednisone | 9.2 | 53.1 | 9.5 | 61.9 | 11.4 | 69.2 | 11.7 | 85.7 | 7.0 |
| Prednisolone | 6.1 | 48.9 | 5.2 | 58.7 | 4.6 | 69.2 | 3.5 | 78.0 | 8.1 |
| Cortisone | 2.1 | 34.4 | 4.9 | 42.7 | 5.1 | 48.6 | 7.0 | 65.6 | 3.4 |
| Boldenone | 0.7 | 35.5 | 4.9 | 52.5 | 1.9 | 40.9 | 12.8 | 65.6 | 5.8 |
| Norethisterone | 2.3 | 22.9 | 5.3 | 31.8 | 5.0 | 16.7 | 7.9 | 49.2 | 5.1 |
| Nandrolone | 4.1 | 25.3 | 8.2 | 41.3 | 3.4 | 24.0 | 4.8 | 59.2 | 5.1 |
| Progesterone | 0.5 | 67.7 | 7.2 | 79.6 | 5.9 | 32.2 | 14.1 | 57.0 | 12.7 |
The repeatability and recoveries were studied intra-day using six samples of contaminated waste water with hormones at low and high concentration levels (100 and 500 ng L−1). These analytical parameters have been studied in samples from the effluent of the tertiary treatment of WWTP1 and in samples from WWTP2, WWTP3 and WWTP4 effluents.
The recoveries calculated are a combination of extraction recoveries and matrix effects on the analytes in the detector due to the impossibility of separating the extraction and identification processes. For most compounds, the recoveries ranged from 50 to 90%, except prednisolone and megestrol acetate that showed recoveries between 120 and 150%, produced by an enhancement of signal from matrix effects. Only diethylstilbestrol and norgestrel presented recoveries below 40%. The waters of WWTP3 and WWTP4 come from a big population and undergo a traditional water treatment, and the recoveries of this waste water were worse than the recoveries of the samples of the other two WWTPs, which work with a membrane bioreactor technology and have a tertiary process to purify the waste water.
The relative standard deviations were satisfactory and similar for most compounds in all samples. At a concentration of 100 ng L−1 the RSD was slightly to moderately higher than that at a concentration of 500 ng L−1. In all cases, the RSDs were lower than 18%.
The limit of detection (LOD) and the limit of quantification (LOQ) for each compound were calculated from the signal to noise ratio of each individual peak. The LOD was defined as the lowest concentration that gave a signal to noise ratio that was greater than 3. The LOQ was defined as the lowest concentration that gave a signal to noise ratio that was greater than 10. The LODs and LOQs ranged from 0.5 to 13.2 ng L−1 and from 1.66 to 44 ng L−1, respectively. These limits are similar to other studies that used off-line SPE with large sample volumes.19,30,31
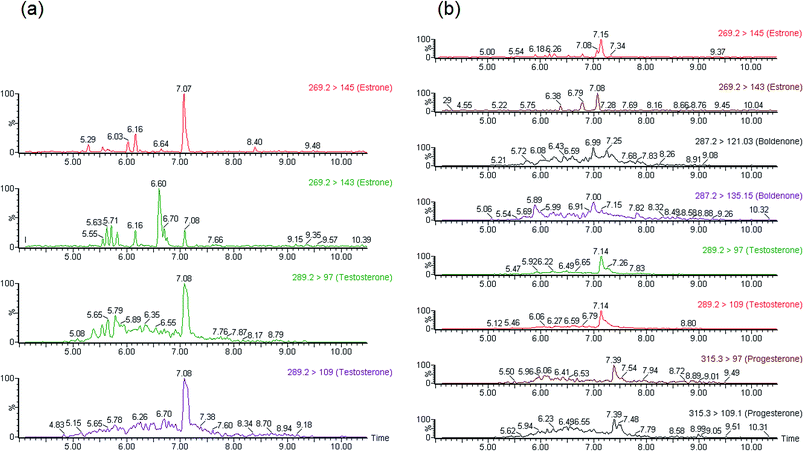
Finally, the method shows a good selectivity as can be seen in Fig. 3. This figure shows the chromatograms of a standard, a non-spiked and a spiked sample.
 | ||
| Fig. 3 Chromatograms of the target compounds in pure solvent (a), a non-spiked real sample (b) and a spiked real sample (c). | ||
3.3. Analysis of selected compounds in waste water samples
The on-line SPE-UHPLC-MS/MS developed method was applied for the detection of target analytes in different waste water samples from WWTPs on the island of Gran Canaria (Spain) to check the efficiency of this method. The samples were collected from the effluent of the tertiary treatment of one WWTP and from effluent of three waste water treatment plants that use a membrane bioreactor and activated sludge as treatments. The samples were collected in May of 2014 and January of 2015. To evaluate the matrix effect, three surrogate standards (estrone D2, testosterone D3 and progesterone D9) were added before the extraction process. They could not be added after the on-line extraction due to the configuration of the UHPLC-MS/MS system. To quantify the concentrations of the compounds, the ratios between the peak area of the quantification ions and the peak area of the surrogate standards were used. Fig. 4a and b show the chromatograms of the compounds found in the effluent samples from WWTP3 and WWTP4.In the effluent of WWTP3, only estrone at a concentration below the quantification limit and testosterone at a concentration of about 50 ng L−1 were detected. In the effluent sample of WWTP4 four steroid hormones were detected. Progesterone and boldenone were detected at concentrations below 5.6 ng L−1, while estrone and testosterone concentrations ranged from 12.6 to 14 ng L−1. The concentrations of each compound found are shown in Table 5.
In the effluent samples from WWTP2, any compound under study that was not detected can be interpreted as a removal of the hormone by the treatment used at WWTP. Several authors have stated this removal in different waste water treatment plants all over the world.13,15,21,32
Table 6 summarizes the studied compounds, sample volume, analysis time and recoveries obtained in other on-line SPE methods used for the determination of steroid hormones in waste water samples. The whole analysis (extraction and determination) usually takes between 10 and 20 minutes as in the method developed in this paper. However, the studies of some authors, Guo et al.18 and Wang et al.,33 present analysis time up to 45 minutes. Another important parameter is the sample volume. In this article, 2 mL of waste water are analyzed, which is a similar volume to that used in other studies by Viglino et al.,32 Ciofi et al.20 and Salvador et al.34 These volumes minimize the analysis time as can be seen in Table 6. The recoveries obtained in this article are in the range of the recoveries obtained by other authors. Nevertheless, the main drawback of other on-line SPE methods is the type of steroid hormone that they determine. Ciofi et al., Wang et al. and Salvador et al.20,33,34 developed on-line SPE methods only for estrogens, while Guo et al., Fayad et al. and Viglino et al.18,28,32 have optimized their methods for estrogens and progestogens or androgens. The on-line SPE method developed in this paper is suitable for estrogens, androgens, progestogens and glucocorticoids. In addition, for the glucocorticoids, this is the first on-line SPE method for their determination, because they have been usually extracted from environmental samples using offline procedures.
| Compounds studied | Sample volume | Analysis time | Average recoveries (%) | Reference |
|---|---|---|---|---|
| Estrogens | 1 mL | 13 min | 79–95 | Salvador et al.34 |
| Estrogens progestogens | 1 mL | 15 min | 85–110 | Viglino et al.32 |
| Estrogens | 50 mL | 45 min | 86–107 | Wang et al.33 |
| Estrogens, progestogens | 10 mL | 15 min | 71–95 | Fayad et al.28 |
| Estrogens androgens | 50 mL | 40 min | 31–120 | Guo et al.18 |
| Estrogens | 2.5 mL | 10 min | 80–98 | Ciofi et al.20 |
| Estrogens, androgens, progestogens, glucocorticoids | 2 mL | 15 min | 43–95 | This study |
4. Conclusions
A selective, sensitive and appropriate on-line SPE-UHLPC-MS/MS method for the determination of hormones in waste water samples at low ng L−1 concentrations was developed. All of the parameters involved in the extraction step, such as the sorbent type, sample volume, loading solvent, wash solvent and pH of the sample, have been optimized to achieve maximum recoveries. The developed method performs a whole process of extraction, identification and determination of four types of steroid hormones in 15 minutes, is fully automated, and requires only 2 mL of water sample; the parallel work with the samples increases efficiency.The developed method offers low limits of detection and quantification (LODs and LOQs ranging from 0.5 to 13.2 ng L−1 and from 1.7 to 44 ng L−1, respectively) and high selectivity, which are important in the analysis of these emerging pollutants in environmental and complex matrices. The recoveries have been satisfactory, ranging between 50 and 90% for most compounds in effluent samples, and all of them with RSDs lower than 15% in most cases.
The application of this method to real samples has been satisfactory and four hormones (one oestrogen, two androgens and one progestogen) have been determined in effluent samples with concentrations ranging from 3 to 52 ng L−1. No hormones were detected in the effluent sample of the waste water treatment plant that uses membrane bioreactor technology.
Acknowledgements
Rayco Guedes-Alonso thanks the University of Las Palmas de Gran Canaria (Spain) for his Ph.D. student grant.References
- J. R. Colman, D. Baldwin, L. L. Johnson and N. L. Scholz, Aquat. Toxicol., 2009, 91, 346–354 CrossRef CAS PubMed.
- P.-D. Hansen, H. Dizer, B. Hock, A. Marx, J. Sherry, M. McMaster and C. Blaise, TrAC, Trends Anal. Chem., 1998, 17, 448–451 CrossRef CAS.
- Ø. Øverli, S. Kotzian and S. Winberg, Horm. Behav., 2002, 42, 53–61 CrossRef.
- J. D. DiBattista, H. Anisman, M. Whitehead and K. M. Gilmour, J. Exp. Biol., 2005, 208, 2707–2718 CrossRef CAS PubMed.
- R. D. Blackburn, A. Cunkelman and V. M. Zlidar, Popul. Rep. A, 2000, 28, 1–16 CAS , 25–32.
- M. Y. Abdel-Rahman and W. W. Hurd, MedScape, 2014, www.medscape.com Search PubMed.
- European Commission, Council Directive 96/22/EC concerning the prohibition on the use in stockfarming of certain substances having a hormonal or thyrostatic action and of beta-agonists, and repealing Directives 81/602/EEC, 88/146/EEC and 88/299/EEC, 1996.
- Y. Xu, N. Xu, N. R. Llewellyn and H. Tao, Environ. Sci.: Processes Impacts, 2014, 16, 262–270 CAS.
- Z. Liu, Y. Kanjo and S. Mizutani, Sci. Total Environ., 2009, 407, 4975–4985 CrossRef CAS PubMed.
- H. Tomšíková, J. Aufartová, P. Solich, L. Nováková, Z. Sosa-Ferrera and J. J. Santana-Rodríguez, TrAC, Trends Anal. Chem., 2012, 34, 35–58 CrossRef PubMed.
- I. Robinson, G. Junqua, R. V. Coillie and O. Thomas, Anal. Bioanal. Chem., 2007, 387, 1143–1151 CrossRef CAS PubMed.
- H. Chang, Y. Wan, S. Wu, Z. Fan and J. Hu, Water Res., 2011, 45, 732–740 CrossRef CAS PubMed.
- G. D'Ascenzo, A. Di Corcia, A. Gentili, R. Mancini, R. Mastropasqua, M. Nazzari and R. Samperi, Sci. Total Environ., 2003, 302, 199–209 CrossRef.
- T. Vega-Morales, Z. Sosa-Ferrera and J. J. Santana-Rodríguez, J. Hazard. Mater., 2010, 183, 701–711 CrossRef CAS PubMed.
- H. Chang, J. Hu and B. Shao, Environ. Sci. Technol., 2007, 41, 3462–3468 CrossRef CAS.
- R. Guedes-Alonso, C. Afonso-Olivares, S. Montesdeoca-Esponda, Z. Sosa-Ferrera and J. J. Santana-Rodriguez, SpringerPlus, 2013, 2, 1–8 CrossRef PubMed.
- N. H. Tran, J. Hu and S. L. Ong, Talanta, 2013, 113, 82–92 CrossRef CAS PubMed.
- F. Guo, Q. Liu, G. Qu, S. Song, J. Sun, J. Shi and G. Jiang, J. Chromatogr. A, 2013, 1281, 9–18 CrossRef CAS PubMed.
- R. Guedes-Alonso, Z. Sosa-Ferrera and J. J. Santana-Rodriguez, J. Anal. Methods Chem., 2013, 2013, e210653 Search PubMed.
- L. Ciofi, D. Fibbi, U. Chiuminatto, E. Coppini, L. Checchini and M. Del Bubba, J. Chromatogr. A, 2013, 1283, 53–61 CrossRef CAS PubMed.
- S. Liu, G.-G. Ying, J.-L. Zhao, F. Chen, B. Yang, L.-J. Zhou and H. Lai, J. Chromatogr. A, 2011, 1218, 1367–1378 CrossRef CAS PubMed.
- S. Rodriguez-Mozaz, M. J. López de Alda and D. Barceló, J. Chromatogr. A, 2004, 1045, 85–92 CrossRef CAS PubMed.
- M. Pedrouzo, F. Borrull, E. Pocurull and R. M. Marcé, Talanta, 2009, 78, 1327–1331 CrossRef CAS PubMed.
- T. Vega-Morales, Z. Sosa-Ferrera and J. J. Santana-Rodríguez, J. Chromatogr. A, 2012, 1230, 66–76 CrossRef CAS PubMed.
- B. Lei, S. Huang, Y. Zhou, D. Wang and Z. Wang, Chemosphere, 2009, 76, 36–42 CrossRef CAS PubMed.
- A. Arditsoglou and D. Voutsa, Mar. Pollut. Bull., 2012, 64, 2443–2452 CrossRef CAS PubMed.
- N. Andrási, B. Molnár, B. Dobos, A. Vasanits-Zsigrai, G. Záray and I. Molnár-Perl, Talanta, 2013, 115, 367–373 CrossRef PubMed.
- P. B. Fayad, M. Prévost and S. Sauvé, Talanta, 2013, 115, 349–360 CrossRef CAS PubMed.
- H. Chang, S. Wu, J. Hu, M. Asami and S. Kunikane, J. Chromatogr. A, 2008, 1195, 44–51 CrossRef CAS PubMed.
- M. Kuster, D. A. Azevedo, M. J. López de Alda, F. R. Aquino Neto and D. Barceló, Environ. Int., 2009, 35, 997–1003 CrossRef CAS PubMed.
- T. Anumol, S. Merel, B. O. Clarke and S. A. Snyder, Chem. Cent. J., 2013, 7, 104 CrossRef PubMed.
- L. Viglino, K. Aboulfadl, M. Prévost and S. Sauvé, Talanta, 2008, 76, 1088–1096 CrossRef CAS PubMed.
- S. Wang, W. Huang, G. Fang, J. He and Y. Zhang, Anal. Chim. Acta, 2008, 606, 194–201 CrossRef CAS PubMed.
- A. Salvador, C. Moretton, A. Piram and R. Faure, J. Chromatogr. A, 2007, 1145, 102–109 CrossRef CAS PubMed.
- Advanced Chemistry Development (ACD/Labs) Software V11.02, Scifinder, Chemical Abstracts Service, Columbus, Ohio, USA, 2013 Search PubMed.
| This journal is © The Royal Society of Chemistry 2015 |