Open Access Article
Open Access ArticleIn situ studies into the characterisation and degradation of blue ballpoint inks by diffuse reflectance visible spectroscopy†
Georgina
Sauzier
,
Peter
Giles
,
Simon W.
Lewis
* and
Wilhelm
van Bronswijk
Department of Chemistry, Curtin University, GPO Box U1987, Perth, Western Australia 6845, Australia. E-mail: S.Lewis@curtin.edu.au; Tel: +61 8 9266 2484
First published on 30th April 2015
Abstract
35 blue ballpoint inks sourced from Western Australian retailers were deposited onto commercial copy paper and analysed using diffuse reflectance visible spectroscopy. Principal component analysis showed that while several pens were clearly distinguishable based on their visible spectra alone, others exhibited overlap. Linear discriminant analysis was then used to build a chemometric model for the classification of the inks. Using a separate validation set, 71.7% of spectra were correctly assigned to a specific pen, and a further 16.7% to the correct pen supplier. Analysis of six pen inks stored under different conditions found the inks remained chemically stable for at least two months when stored in the dark. However, two inks exhibited spectral changes within one week under ambient light, and all but one ink displayed changes within two months, resulting in altered predictions using the chemometric model. This may be useful in cases of alleged fraud, where it is suspected that an ink entry may have been altered using the same pen at a later date. Artificial ageing experiments found that both heat and ultraviolet light play a role in the ageing process, and that accelerated ageing using these factors gives a reasonable depiction of short-term ageing under natural conditions.
Introduction
Despite the increasing trend toward electronic communication and transactions, written documents are still widely used in financial, legal and personal matters.1 The analysis and comparison of pen inks thus remains a highly relevant aspect of forensic investigations.Modern pen inks are complex mixtures consisting of several dyes or pigments carried in a solvent such as water, oil or glycols.2,3 In the case of ballpoint inks, these dyes may constitute up to 50% of the total ink formulation, and are generally contained in either a glycol-based solvent or benzyl alcohol.4,5 Additional components (collectively referred to as the ‘vehicle’) may also include fatty acids, softeners and polymeric resins, which are designed to improve the consistency, flow or drying characteristics of the ink.3,4 This complexity, while potentially making inks a challenging material to analyse, also makes them good candidates for discrimination based on their different (and often proprietary) formulations.
Guidelines such as those set out by the American Society for Testing and Materials (ASTM), or the Scientific Working Group for Forensic Document Examination (SWGDOC), outline a range of analytical methods suited to the analysis of writing inks.6,7 Techniques such as thin layer chromatography (TLC), gas chromatography (GC) or high performance liquid chromatography (HPLC) are frequently utilised in order to separate and identify individual ink components.8–10 In recent years, infrared and Raman spectroscopy have gained popularity as ideal techniques for providing rapid and non-destructive information regarding the structure of dyes or other organic components, without extensive sample preparation.11,12
Significant research has also been carried out using ultraviolet (UV) or visible spectroscopy and microspectrophotometry (MSP).13–15 Patented ink formulations often contain unique combinations of dyes and pigments, making colour a highly discriminating characteristic. In a similar fashion to infrared and Raman spectroscopy, these methods – when used in situ – also have the advantages of being rapid, non-destructive and requiring little to no sample preparation.
Recent studies have employed visible spectroscopy and MSP with chemometrics to provide more objective assessments over visual inspections, and allow the simultaneous comparison of several samples at a time.16–19 In the majority of these studies, however, inks were extracted into ethanol rather than being analysed on a paper substrate. As well as being destructive, the analysis of solvent extracts may not give a realistic representation of spectra acquired from ink on paper.
Another important consideration is the ageing of ink deposited on a substrate. Once exposed to the environment, compositional changes may rapidly occur due the loss of volatile solvents, photofading of dyes or the polymerisation of resins.20–23 The precise rates at which these processes occur are highly dependent on a number of factors, including the initial ink composition, paper type and storage conditions.24–26 This poses a significant challenge to document examiners when attempting to compare ink entries of different ages, as ink from the same pen may give rise to very different spectral profiles.
Several studies have examined both the natural and artificial ageing of inks, with the view of developing reliable dating methods.27 However, there is limited research in the open literature considering the effects of ageing on inks characterised using chemometric methods.
Senior et al. investigated the analysis of blue ballpoint inks using UV-visible spectroscopy, with subsequent characterisation using PCA. They found that inks analysed after 2–18 months were easily distinguishable from fresh samples, and postulated that these changes could be correlated with time to estimate when a questioned document was written.28 However, this study employed a limited sample set of only 10 pens, with the inks again analysed as extracts rather than on a paper substrate.
This paper presents initial results of an on-going study into the discrimination of blue ballpoint inks on paper using visible spectroscopy with chemometric modelling, and how these models may be affected by ink ageing under typical storage conditions. Artificial ageing experiments were also conducted to determine the contribution of specific environmental factors to the ageing process, and whether artificial ageing provides an adequate simulation of natural ageing processes under the selected conditions.
Materials and methods
Sample collection and preparation
35 blue ballpoint pens were obtained from existing stationery supplies and various Western Australian retailers (refer to ESI Table S1†). Ink from each pen was deposited onto white copy paper (Fuji Xerox Professional Carbon Neutral, 80 g m−2) by filling 10 mm × 10 mm squares using parallel lines (Fig. S1†). Five replicate samples were prepared using each pen to account for any inhomogeneity within the ink cartridge.An additional five replicates were prepared from 12 pens selected as a validation set (Table S1†). Where pens had been purchased as a packet, a different pen from the same packet was randomly selected to prepare the validation samples.
Ageing studies
Inks from six newly purchased pens were selected to undergo both natural and artificial ageing. Due to space limitations, it was deemed impractical to prepare five replicate ageing samples of each pen for each set of storage parameters. Instead, larger samples (25 mm × 25 mm) were prepared to allow replicate measures to be made from different locations on each sample.For natural ageing investigations, samples were stored in three different typical storage environments, with analysis at various intervals following ink deposition (1 day, 1 week, 2 weeks, 1 month, and 2 months):
(i) On an office shelf, exposed to ambient light and air.
(ii) In an office drawer, away from light but exposed to air.
(iii) In an office drawer, away from light and stored in plastic archive sleeves (Ditto A4 reinforced sheet protectors).
Thermal ageing was conducted by placing samples into a ZhiCheng ZXRD-A5055 oven at 100 °C. The samples were analysed after 20 minutes, 2 hours and 24 hours of exposure. Aluminium foil was placed over the glass oven window to prevent UV exposure of the samples during this period.
UV accelerated ageing was conducted via irradiation with a compact fluorescent UV light (20 W, Nelson Industries, Australia, model MELS20BLKES) mounted overhead on a Firenze Mini Repro copy stand. Samples were placed approximately 17 cm below the light source and analysed following 24 hours and 48 hours of UV exposure.
All artificial ageing experiments were carried out under controlled air-conditioning; however, as a climate control chamber was not available during these studies, no further attempts were made to control factors such as ambient humidity. Data collected using a Digitech QP-6013 data logger found that during these experiments, the relative humidity remained reasonably constant (50–60%).
Visible spectroscopy
Spectra were obtained using a Cary 4000 UV-Visible spectrophotometer equipped with a DRA-900 internal diffuse reflectance accessory. Baseline scans were taken using an empty sample holder and mounted halon reference plate prior to sample measurement. The instrument was operated with a reduced slit height in double beam mode, and data acquisition performed using the Cary WinUV Bio Version software (v. 4.20). Spectra were recorded over the range of 400–700 nm, with a scan interval of 1 nm and scan speed of 600 nm s−1.Data analysis
All data pre-processing and chemometric analysis was performed using the Unscrambler® X software (v. 10.3, Camo Software AS, Oslo, Norway). Two pre-processing sequences were examined. Spectra were first baseline corrected and unit vector normalised to remove variability caused by the sample surface texture or amount of ink deposited. In the first pre-processing approach, spectra were then adjusted to a maximum reflectance value of 1 and converted using the Kubelka–Munk function. In the second approach, chemometric analysis was conducted immediately after normalisation without further pre-processing.Principal component analysis (PCA) was performed on the initial spectra from each of the 35 ballpoint pens, and the samples plotted using the first three principal components (PCs). This was used to visualise the distribution of the samples, and identify any outliers.
A predictive model was then built from the same spectral dataset using linear discriminant analysis (LDA) and data derived from PCA. This calibration model was used to predict spectra from the validation set (12 pens), with the actual vs. predicted classifications compared to evaluate the efficacy of the model. The model was also used to predict spectra from both the natural and artificial ageing samples. The original and predicted classifications were compared to detect any changes in the deposited inks over time.
Results and discussion
Preliminary considerations
The results obtained through chemometric analysis are largely dependent upon the initial pre-processing of the data.29 For this study, two different approaches were thus examined. In the first instance, a Kubelka–Munk (K–M) conversion was performed. This function is frequently applied to diffuse reflectance spectra to allow quantitative information to be derived, which may be useful for discrimination purposes.30,31 The spectra were adjusted to a maximum reflectance of 1 prior to conversion, as the K–M function poorly handles values approaching zero, such as those obtained through normalisation.It was found that the K–M function did not result in any improved overall discrimination between samples. In fact, the classification accuracy determined using the validation set decreased by 10% compared to results obtained with only a baseline correction and normalisation. This may be due to the amplification of deviations which may be observed when reflectance spectra are converted to K–M units.30 For this reason, the K–M function was omitted in all further data analysis. The following discussion is presented in reference to results obtained without K–M conversion.
Distribution of sample set
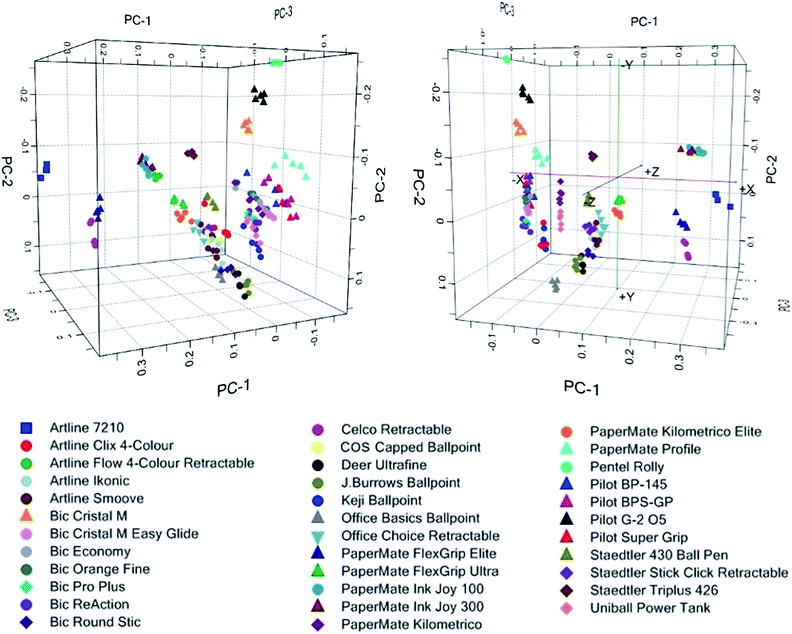
PCA was employed to reduce the dimensionality of the data, by transforming the original variable set into a lesser number of latent orthogonal variables known as principal components.32–34 By assessing the first few PCs, which account for the majority of variation in the dataset, it may be possible to establish relationships or patterns between samples which would not be readily evident from the raw data.34Spectra from the 35 ballpoint pen inks were plotted using the first three PCs (accounting for 95.8% of total variance) as a new coordinate system, resulting in the scores plot shown in Fig. 1. Replicate samples from each individual pen were clustered closely together, indicating good reproducibility in the sampling and analysis method. Several inks formed visually distinct groupings, allowing rapid discrimination based solely upon their visible spectra. In some cases, these spectra appeared visually very similar (Fig. 2), and may have been difficult to distinguish based on visual comparison. This example highlights the discriminative power associated with chemometric techniques, which makes them well suited to forensic purposes.
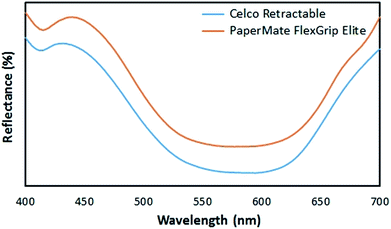 | ||
| Fig. 2 Raw diffuse reflectance visible spectra obtained from Celco Retractable and PaperMate FlexGrip Elite inks on copy paper. Spectra have been offset for visual clarity. | ||
Despite many of the pens being visually distinctive, a number of overlapping clusters were observed in which one or more pens could not be clearly distinguished (Table 1). PCA repeated on these individual clusters failed to improve the separation between these samples.
| Cluster | Pens |
|---|---|
| Group 1 | Bic Pro Plus, Bic ReAction |
| Group 2 | Bic Cristal M Easy Glide, Bic Economy, Bic Orange Fine |
| Group 3 | Pilot BP-145, Pilot BPS-GP, Pilot Super Grip |
| Group 4 | Artline Ikonic, Artline Smoove, COS Capped Ballpoint, Office Choice Retractable, Staedtler Stick Click Retractable |
| Group 5 | Artline Flow 4-Colour Retractable, PaperMate Ink Joy 100, PaperMate Ink Joy 300 |
For groups 1–3, which all consisted of pens derived from the same brand (supplier), the overlap between these samples is most likely due to suppliers using the same ink formulation across a range of different pens. Groups 4 and 5, however, contained a mixture of pens from different brands. This is possibly due to these suppliers coincidentally using similar ink formulations. However, it should be noted that although certain suppliers such as Bic© produce their own ink systems,35 many suppliers source their inks externally, and a distinction must be made between the ‘supplier’ and ‘manufacturer’ of any given ink. Thus, it is also possible that budget suppliers such as COS or Office Choice may have purchased inks from the same manufacturers as larger suppliers such as Artline and PaperMate. This could result in the same ink being used by different suppliers, due to a common manufacturer.
Discriminant analysis
LDA was performed using data derived from the PCA. LDA is a technique that establishes classification rules for pre-specified groupings, such that maximum discrimination is achieved between them.33,36,37 The subsequent model can then be employed to assign unknown samples to the most probable class.As previously mentioned, the calibration model was built using the same spectral dataset utilised in PCA, with the efficacy of the model evaluated using a separate validation set comprising 12 pens. Six of these pens were deliberately chosen as exhibiting overlap or close clustering with other pens in the PCA plot, to see how the model would handle these samples.
LDA was conducted using the Mahalanobis distance and first four PCs (accounting for 98.0% of total variance). The fourth PC, accounting for 2.2% of total variance, was found to provide additional discrimination between the Pilot brand pens. The resultant model successfully classified 97.7% of the data in the calibration set (Table S2†), and 71.7% of data in the validation set (Table 2). It should be noted that using the same data to both build and test a model can lead to over-optimistic predictions of its performance, hence the discrepancy in results obtained between the calibration and validation sets.29,37
| Pen | Correct | Incorrect | % Correct |
|---|---|---|---|
| Bic Cristal M | 5 | 0 | 100 |
| Office Basics Ballpoint | 5 | 0 | 100 |
| J.Burrows Ballpoint | 5 | 0 | 100 |
| Bic Economy | 0 | 5 (predicted as Bic Orange Fine) | 0 |
| Uniball Power Tank | 4 | 1 (predicted as Bic Cristal M) | 80 |
| Pilot BPS-GP | 4 | 1 (predicted as Pilot BP-145) | 80 |
| Bic Pro Plus | 2 | 3 (predicted as Bic ReAction) | 40 |
| PaperMate Flexgrip Elite | 3 | 2 (predicted as J.Burrows Ballpoint) | 60 |
| Pentel Rolly | 5 | 0 | 100 |
| Staedtler Triplus 426 | 4 | 1 (predicted as Staedtler Stick Click) | 80 |
| PaperMate Ink Joy 300 | 5 | 0 | 100 |
| Office Choice Retractable | 1 | 4 (predicted as Staedtler Stick Click) | 20 |
| Total | 43 | 17 | 71.7 |
As expected, most incorrect classifications were obtained against inks that exhibited overlap in the PCA. For example, ink from the Bic Economy was predicted as originating from a Bic Orange Fine. Interestingly though, ink from the Office Basics ballpoint and PaperMate Ink Joy 300 were correctly predicted, despite being very similar to other pens in the population. Additionally, 16.7% of spectra, while not correctly assigned to a specific pen, were assigned to the correct pen supplier.
With this in mind, the use of visible spectroscopy with chemometrics shows good potential for the discrimination (and possible identification) of blue ballpoint inks on paper. The developed model may also be applicable as a screening method, and could be used to exclude particular inks from further investigation.
Characterisation of naturally aged samples
Inks from six newly purchased pens were left to undergo natural ageing under three different conditions, with analysis at intervals ranging between 1 day and 2 months after deposition.Following two months of exposure, ink samples stored in the dark (both open to air or contained in plastic archive sleeves) were still correctly predicted using the chemometric model. Projection of these samples onto the PCA plot found that the aged inks still largely clustered with their expected pens (Fig. S2 and S3†). However, a larger amount of intra-group variation was observed in inks which were left open to air, indicative of a gradual ageing effect potentially due to the slow evaporation of solvents. In particular, aged ink from the Celco Retractable and PaperMate Profile pens appear to be shifting away from their freshly deposited counterparts.
Misclassifications were observed in these same pens within just one week of exposure to light in addition to open air. Over the following analysis intervals, all pens except for the Pentel Rolly (Pen 26) eventually gave incorrect predictions, as shown in Table 3. It was found that most of the inks aged at different rates, with the first observable spectral changes occurring at different points within the two-month interval. This is consistent with findings in previously mentioned studies, which found that ageing rates are at least partially dependent on the initial ink composition, such as the volatility of solvents or the stability of the various dyes and pigments.24–26
| Time since deposition | Pen 8 (Celco Retractable) | Pen 10 (Keji Ballpoint) | Pen 11 (Office Basics) | Pen 19 (Pilot G-2 05) | Pen 23 (PaperMate Profile) | Pen 26 (Pentel Rolly) |
|---|---|---|---|---|---|---|
| 1 day | 0 | 0 | 0 | 0 | 0 | 0 |
| 1 week | 5 (Pen 13) | 0 | 0 | 0 | 5 (Pen 10) | 0 |
| 2 weeks | 5 (Pen 13) | 3 (Pen 24) | 1 (Pen 13) | 0 | 3 (Pen 10) | 0 |
| 2 (Pen 18) | 1 (Pen 20) | |||||
| 1 (Pen 22) | ||||||
| 1 month | 5 (Pen 13) | 2 (Pen 24) | 4 (Pen 13) | 0 | 3 (Pen 1) | 0 |
| 3 (Pen 18) | 1 (Pen 20) | |||||
| 1 (Pen 22) | ||||||
| 2 months | 5 (Pen 13) | 5 (Pen 11) | 5 (Pen 13) | 5 (Pen 22) | 1 (Pen 1) | 0 |
| 3 (Pen 20) | ||||||
| 1 (Pen 22) |
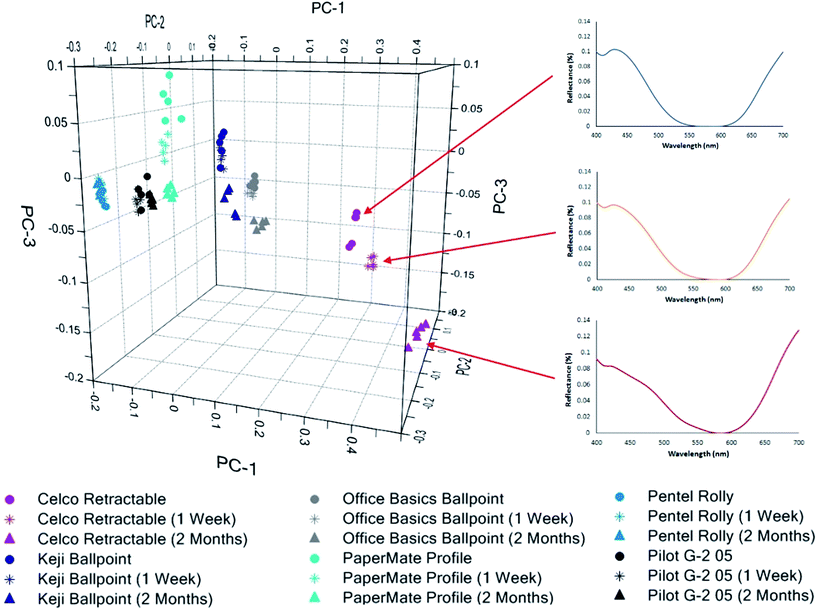
Projection of the aged inks onto the original PCA scores plot gives a clear visual indication of these changes, as well as the different ageing rates (Fig. 3). As expected, the largest spread between the fresh and aged ink were observed for the Celco Retractable and PaperMate Profile, which were the first inks to become misclassified by the model and can be considered as ‘fast-ageing’. In contrast, ‘slow-ageing’ samples such as the Pilot G-2 05 and Pentel Rolly are still clustered with their fresh ink equivalents, with no visible change discernible in their spectra over the ageing period.
Spectra from the Celco Retractable ink over time show that while the broad shape of the spectra have remained the same, the relative reflectance has decreased in the blue region (c.a. 430 nm) and increased in the red region (700 nm), as seen in Fig. 3. Inspection of the PCA factor loadings (Fig. S4†) allows these changes to be related to the shift in these spectra on the scores plot. It can be seen that PC1 exhibits a strong positive correlation at 700 nm, and a negative correlation between 400–500 nm. Hence, as the reflectance values decrease in the 400 nm region and increase in the 700 nm region, the scores attained along PC1 become more positive, as observable in Fig. 3.
Similarly, it was noted that spectra from the PaperMate Profile ink obtain more positive scores along PC3 over the two month period. PC3 exhibits a negative correlation at ca. 410 nm, and a positive correlation at 450 nm, which are again consistent with changes noted in the spectra of this ink over the ageing period (Fig. S5†).
For both the Celco and the PaperMate inks, visual inspection of the ink deposits showed no apparent change in appearance that could be readily discerned by eye, despite the visible change in the MSP spectra. This highlights the necessity of objective colour measurement techniques when conducting forensic comparisons, as instrumental techniques are capable of detecting changes that are not readily visible to the naked eye.
Due to the proprietary nature of the ink formulations, and the lack of structural information discernible from visible spectra, it cannot be said with certainty which ink components are responsible for the observed ageing effects. However, as these changes were limited to samples exposed to light, it is a reasonable assumption that they can be attributed to photofading of the dyes.
The most commonly utilised dyes in modern blue ballpoint inks are synthetic triarylmethane dyes, such as methyl violet and crystal violet, which are known to have low photostability.38 The typical degradation pathway for these structures occurs through demethylation, as well as loss of conjugation due to opening of the aromatic ring.22,39 These photocatalysed processes are known to be accelerated by the presence of titanium dioxide; a filler commonly utilised in the paper industry to impart high opacity and brightness.40
Spectral changes due to ageing pose an obvious issue in applying chemometric models to ‘real’ samples, which may be several weeks to years old. The rapid alteration of visible spectra following ink deposition may result in ink entries from the same pen being falsely excluded due to different age or storage history. Incorrect information could also be generated when attempting to predict (identify) an aged ink on the basis of its visible spectrum.
Fortunately, such samples can be identified as atypical through their discriminant values; distance measures between an unknown sample and the centroid of each known group. Assignment is made to the group resulting in the smallest magnitude values, indicative of the ‘closest fit’. Table 4 shows the discriminant values obtained by the aged Celco Retractable ink for the J.Burrows pen, to which the ink was incorrectly classified. However, the values against this group are several orders of magnitude larger than those from actual J.Burrows ink, demonstrating that the aged ink is not well classified. This example illustrates the importance of considering discriminant values before accepting classifications given using LDA.
| Pen | Mean discriminant value | Std deviation |
|---|---|---|
| J.Burrows Ballpoint | −3.20 | 0.00 |
| Celco Retractable – 1 week | −7869.37 | 991.94 |
| Celco Retractable – 2 weeks | −9056.74 | 1469.80 |
| Celco Retractable – 1 month | −12077.99 | 1612.34 |
| Celco Retractable – 2 months | −11784.83 | 2263.38 |
Although the rapid ageing of deposited inks may prove problematic in some scenarios, in other instances it may in fact be beneficial. In cases of suspected fraud or forgery, it is often of interest to determine whether an ink entry could have been modified at a later date. This may include the supposed contemporaneous signing of a will by multiple parties, or the suspected alteration of banking cheques. These analyses may be problematic if changes have been made using the same pen as the original entry. However, the results here suggest that entries completed using the same pen, as little as one week apart, may be differentiated if the document has been exposed to light during the intervening period.
Characterisation of artificially aged samples
Artificial ageing experiments were carried out to examine the individual effects of heat and UV exposure on ink degradation, and determine whether the induced changes provided a realistic comparison with naturally aged inks exposed to light and air.Samples of the six inks utilised in the natural ageing study were heated in an oven at 100 °C as recommended by Cantu, who theorised that four minutes at this temperature would provide an equivalent ageing to three months under natural conditions.41 After 20 minutes to 2 hours of heat exposure, ink from the PaperMate Profile pen was misclassified as originating from a Keji Ballpoint, while three spectra from the Celco Retractable ink were incorrectly attributed to a J.Burrows Ballpoint after 24 hours. In this scenario, these results are consistent with changes observed in the first week of natural ageing under ambient light.
Grim et al. suggested that during the demethylation of dyes such as methyl violet, solvent molecules provide protons to replace the lost substituents.24 Thus, while a thermal approach may be suitable for representing changes in the ink's solvent content, the removal of solvent may hinder degradation of the dye molecules, thus resulting in little change to the visible spectrum of the ink. This supports conclusions from the natural ageing study that dye degradation is the key influence in the observed ageing effects. Nonetheless, the changes noted in thermally treated samples despite the absence of light indicates that solvent loss is also a relevant factor.
UV accelerated ageing was conducted by irradiating ink samples under a UV lamp. Inks from the PaperMate Profile and Celco Retractable pens were misclassified as Keji and J.Burrows inks respectively, following 24 hours of exposure. These results are again consistent with early changes noted in samples aged naturally for one week under open conditions. Interestingly, no further misclassifications were observed between 24 and 48 hours of irradiation. Siegel postulated that that the loss of methyl groups due to UV exposure yields stable products that resist further degradation.42 However, given the continuing changes in naturally exposed inks, it is perhaps more likely that the photodegradation of dyes is partially due to visible light rather than solely UV. Previous studies have achieved decolourisation of crystal violet and other triarylmethane dyes using visible irradiation in the presence of zinc oxide or titanium oxide.39,43
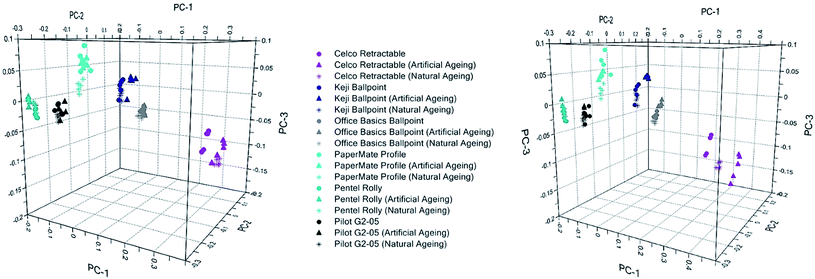
Fig. 4 provides a comparison of the inks artificially aged for 24 hours and those aged naturally for one week, compared to freshly deposited ink. It can be seen that inks aged by UV show a greater shift away from their fresh counterparts compared to those that were thermally aged. In both instances, however, the direction of these shifts is consistent with naturally aged samples. Spectral changes in the artificially aged samples are also consistent with those obtained through natural ageing (Fig. S5–S7†). Hence, either approach can be considered to give a realistic depiction of natural ageing processes.
Conclusions
The overall results of this study indicate that diffuse reflectance visible spectroscopy with chemometrics provides a simple, rapid and non-destructive method for studying the discrimination of blue ballpoint inks deposited on paper. It should be noted that the large ink deposits prepared in this study are unlikely to be representative of real samples such as handwriting, and thus in a casework scenario, equivalent visible spectra would be acquired using a microspectrophotometer due to its smaller sample aperture.Using a validation set of 12 pens, 88.3% of spectra could be correctly assigned to either the individual pen type or the pen brand. The developed model may hence be applicable as a screening method to rapidly compare multiple pen inks at a time, and exclude those which are dissimilar. Additionally, prediction using LDA may allow the identification of an unknown ink where a known reference is unavailable, or where comparison with another sample is not of primary interest. However, as some pens proved to be difficult to separate on the basis of colour alone, further studies may determine whether alternative techniques such as infrared or Raman spectroscopy provide additional discrimination, by characterising non-colourant components such as solvents or resins.
Analysis of six pen inks stored under natural ageing conditions determined that inks stored in the dark could still be reliably predicted for up to at least two months. However, inks stored in the light could exhibit significant changes to their spectral profile within just one week, which may prove useful during investigations of suspected fraud. This indicates that the photofading of dyes and/or pigments is a key factor in the early ageing process. No visual colour change in the aged inks were found over an initial two month period; however, it is anticipated that further study may reveal subsequent changes over a long-term ageing period.
Artificial ageing using both thermal and UV irradiation methods confirm the findings of the natural ageing study, with UV exposed inks exhibiting greater change than heat-treated samples over an identical time period. However, the successful ageing of inks using either method suggests that both factors play a role in the ageing process. Changes induced through artificial ageing were also found to be comparable with those obtained via short-term natural ageing under open conditions.
Based upon previous literature, it is hypothesised that triarylmethane dyes present in the inks may be undergoing photocatalysed demethylation, accelerated by the presence of titanium dioxide in the paper substrate. However, this cannot be stated with certainty as the compositions of the studied inks are currently unknown. Therefore, further investigation using techniques such as TLC or GC-MS are warranted to identify the constituents of the ink which may be of specific interest. Additionally, as noted, one factor that has not yet been investigated is the potential effect of humidity, which may also be the subject of future work.
The present study employed a very specific range of pen inks on a single substrate, all sourced from Western Australia. Additional studies involving a range of ink types (such as gel, roller-ball and felt-tip varieties) on different substrates (including coloured paper) could be conducted to determine whether similar results can be achieved. The sample set could also be expanded to incorporate international suppliers, to allow the potential application of this model to a broader geographical context.
Additionally, ink formulations are constantly being re-designed to improve characteristics such as colour and viscosity. As with any forensic database, this will hence necessitate the consistent addition of new data through continued sample collection and/or international collaboration.
Acknowledgements
The authors thank Peter Chapman for providing technical assistance with visible spectroscopy. Portions of this work were presented in the inaugural RSC Analytical Science Twitter Poster Conference (5th Feb. 2015). Georgina Sauzier is supported by an Australian Postgraduate Award.Notes and references
- T. Trubshoe and J. McGinn, in Encyclopedia of Forensic Sciences, ed. M. M. Houck, J. A. Siegel and P. J. Saukko, Academic Press, Waltham, 2013, pp. 360–366 Search PubMed.
- M. M. Houck and J. A. Siegel, Fundamentals of Forensic Science, Academic Press, Burlington, MA, 2nd edn, 2010 Search PubMed.
- S. Bell, Encyclopedia of Forensic Science, Infobase Publishing, New York, 2008 Search PubMed.
- J. A. Siegel, in Encyclopedia of Forensic Sciences, ed. M. M. Houck, J. A. Siegel and P. J. Saukko, Academic Press, Waltham, 2nd edn, 2013, pp. 375–379 Search PubMed.
- R. A. Merrill and E. G. Bartick, J. Forensic Sci., 1992, 37, 528–541 Search PubMed.
- Scientific Working Group for Forensic Document Examination, 2013.
- American Society for Testing and Materials, 2004.
- A. A. Kher, E. V. Green and M. I. Mulholland, J. Forensic Sci., 2001, 46, 878–883 CAS.
- B. Li, P. Xie, Y.-m. Guo and Q. Fei, J. Forensic Sci., 2014, 59, 543–549 CrossRef CAS PubMed.
- C. Neumann, R. Ramotowski and T. Genessay, J. Chromatogr. A, 2011, 1218, 2793–2811 CrossRef CAS PubMed.
- A. Braz, M. López-López and C. García-Ruiz, Forensic Sci. Int., 2014, 245, 38–44 CrossRef CAS PubMed.
- Y. S. Nam, J. S. Park, Y. Lee and K.-B. Lee, J. Forensic Sci., 2014, 59, 800–805 CrossRef CAS PubMed.
- D. L. Feraru, M. Mihaly and A. Meghea, Color Res. Appl., 2015, 40, 169–177 CrossRef.
- C. Roux, M. Novotny, I. Evans and C. Lennard, Forensic Sci. Int., 1999, 101, 167–176 CrossRef.
- S.-K. Shon, H.-S. Park, J.-S. Lee, S.-W. Park and S.-H. Cho, Anal. Sci. Technol., 2000, 13, 250–257 CAS.
- C. D. Adam, S. L. Sherratt and V. L. Zholobenko, Forensic Sci. Int., 2008, 174, 16–25 CrossRef CAS PubMed.
- D. Ismail, Z. Austad and W. N. S. M. Desa, Malaysian Journal of Forensic Sciences, 2008, 5, 47–52 Search PubMed.
- V. A. G. da Silva, M. Talhavini, I. C. F. Peixoto, J. J. Zacca, A. O. Maldaner and J. W. B. Braga, Microchem. J., 2014, 116, 235–243 CrossRef CAS.
- N. C. Thanasoulias, N. A. Parisis and N. P. Evmiridis, Forensic Sci. Int., 2003, 138, 75–84 CrossRef CAS PubMed.
- J. Andrasko, J. Forensic Sci., 2001, 46, 21–30 CAS.
- S. Lociciro, L. Dujourdy, W. Mazzella, P. Margot and E. Lock, Sci. Justice, 2004, 44, 165–171 CrossRef CAS PubMed.
- C. Weyermann, D. Kirsch, C. Costa-Vera and B. Spengler, J. Am. Soc. Mass Spectrom., 2006, 17, 297–306 CrossRef CAS PubMed.
- C. Weyermann, D. Kirsch, C. C. Vera and B. Spengler, Forensic Sci. Int., 2007, 168, 119–127 CrossRef CAS PubMed.
- D. M. Grim, J. Siegel and J. Allison, J. Forensic Sci., 2002, 47, 1–9 Search PubMed.
- A. Koenig, J. Bügler, D. Kirsch, F. Köhler and C. Weyermann, J. Forensic Sci., 2015, 60, S152–S161 CrossRef CAS PubMed.
- C. Weyermann and B. Spengler, Forensic Sci. Int., 2008, 180, 23–31 CrossRef CAS PubMed.
- M. Ezcurra, J. M. G. Góngora, I. Maguregui and R. Alonso, Forensic Sci. Int., 2010, 197, 1–20 CrossRef CAS PubMed.
- S. Senior, E. Hamed, M. Masoud and E. Shehata, J. Forensic Sci., 2012, 57, 1087–1093 CrossRef CAS PubMed.
- R. G. Brereton, in Applied Chemometrics for Scientists, John Wiley & Sons, Chichester, England, 2007, pp. 145–191 Search PubMed.
- Y.-Z. Liang, A. A. Christy, A. K. Nyhus, S. Hagen, J.-S. Schanche and O. M. Kvalheim, Vib. Spectrosc., 1999, 20, 47–57 CrossRef CAS.
- E. Péré, H. Cardy, O. Cairon, M. Simon and S. Lacombe, Vib. Spectrosc., 2001, 25, 163–175 CrossRef.
- P. J. Gemperline, in Practical Guide to Chemometrics, CRC Press, Boca Raton, Florida, 2nd edn, 2006, pp. 69–104 Search PubMed.
- V. Kinton, in Practical Analysis of Flavor and Fragrance Materials, John Wiley & Sons, 2011, pp. 91–110 Search PubMed.
- K. Varmuza and P. Filzmoser, in Introduction to Multivariate Statistical Analysis in Chemometrics, Taylor & Francis, Boca Raton, Florida, 2009 Search PubMed.
- How it is Manufactured - Stationery, http://www.bicworld.com/en/products/how-its-manufactured-stationery/, accessed March 11, 2015.
- H. Bensmail and G. Celeux, J. Am. Stat. Assoc., 1996, 91, 1743–1748 CrossRef.
- J. N. Miller and J. C. Miller, Statistics and Chemometrics for Analytical Chemistry, Pearson Education, Harlow, England, 6th edn, 2010 Search PubMed.
- D. Thetford, in Kirk-Othmer Encyclopedia of Chemical Technology, ed. A. Seidel and M. Bickford, John Wiley & Sons, New Jersey, 2013 Search PubMed.
- X. Li, G. Liu and J. Zhao, New J. Chem., 1999, 23, 1193–1196 RSC.
- P. Bajpai, Environmentally Friendly Production of Pulp and Paper, John Wiley & Sons, New Jersey, 2010 Search PubMed.
- A. A. Cantu, J. Forensic Sci., 1988, 33, 744–750 Search PubMed.
- J. Siegel, J. Allison, D. Mohr and J. Dunn, Talanta, 2005, 67, 425–429 CrossRef CAS PubMed.
- M. A. Habib, M. Muslim, M. T. Shahadat, M. N. Islam, I. M. I. Ismail, T. S. A. Islam and A. J. Mahmood, J. Nanostructure Chem., 2013, 3, 1–10 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ay00761e |
| This journal is © The Royal Society of Chemistry 2015 |