Open Access Article
Open Access ArticleEvaluation of different functional groups for covalent immobilization of enzymes in the development of biosensors with oxygen optical transduction†
Teresa
Ramon-Marquez
a,
Antonio L.
Medina-Castillo
*b,
Jorge F.
Fernandez-Sanchez
*a and
Alberto
Fernández-Gutiérrez
a
aDepartment of Analytical Chemistry, University of Granada, Avda. Fuentenueva s/n, 18071 Granada, Spain. E-mail: jffernan@ugr.es; Fax: +34 958243328; Tel: +34 958240451
bNanoMyP®, Nanomateriales y Polimeros S.L., Spin-Off Company of the UGR, BIC building, Avd. Innovacion 1, E-18016, Granada, Spain. E-mail: amedina@nanomyp.com
First published on 9th February 2015
Abstract
Four different types of polymeric particles with different functional groups on their surface have been evaluated to develop biosensors using glucose oxidase as a model enzyme. Direct covalent immobilization was achieved on particles functionalized with chloride, epoxy and vinyl groups via the reactive functional groups on the surface, whereas particles functionalized with carboxylic groups, used as reference materials, were pre-activated with carbodiimide. Immobilization was successfully performed under very mild conditions (20 °C, pH 8.0). In order to determine the advantages and disadvantages of each functional group, both the amounts of immobilized enzymes and their relative activities were fully investigated. In order to demonstrate their applicability on the design on biosensing with oxygen optical transduction, the functionalized particles were co-immobilized in gold chips with oxygen sensing particles (PSMA–PtTFPP) using electrophoretic deposition and characterised for glucose determination in solution.
Introduction
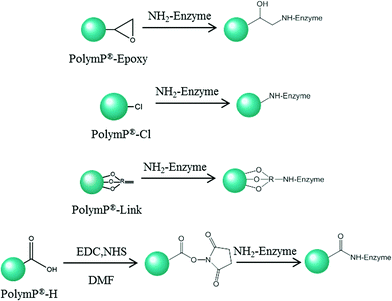
To develop a biosensing film with optical oxygen transduction, two elements, at least, are necessary:1 (1) the biochemical recognition element (i.e. oxidase enzyme) which can oxidise the analyte and change the concentration of oxygen in the media, and (2) an oxygen transduction element which provides the analytical signal.Immobilization of biomolecules and the preservation of their biological activities have proven particularly valuable and have been exploited to enhance their biomolecule properties for successful utilization in the development of biosensors.2 The vast amount of coupling chemistry and the diversity of the carrier structure are also powerful assets for modulating the catalytic properties of the enzyme. Thus, selection or preparation of the carrier is of crucial importance with regard to the performance of the immobilized enzymes.3,4 Herein, four different types of spherical polymeric particles with different functional groups on their surface were tested for covalent immobilization of GOx, which was used as a model enzyme: chloride groups (PolymP®-Cl), epoxy groups (PolymP®-Epoxy), particles with a pre-activated vinyl surface (PolymP®-Link) and classical particles functionalized with carboxyl groups (PolymP®-H) were used as reference materials. Optimum immobilization conditions of GOx for each type of particle were investigated.
The oxygen sensitive transducer should consist of an oxygen sensitive dye incorporated into an oxygen permeable, inert support. Pt(II) meso-tetra(pentafluorophenyl)porphine (PtTFPP) is one of the most widely used dyes for the preparation of optical oxygen sensing films due to its good photostability,5 long luminescence lifetime, wide excitation range, and large Stokes shift, which simplifies the measurement system.6,7 On the other side, polymeric materials have demonstrated good properties in terms of solubility of the dye and permeability to oxygen.8,9 It has been proved that poly(styrene-co-maleic anhydride) provides good stable and high zeta potential nanoparticles.10,11 Thus, PSMA–PtTFPP was used as optical oxygen transducers in this work.
Finally, these two elements have been deposited on an inert support in order to demonstrate their applicability in the determination of the analyte in solution. There are several strategies to deposit polymeric nanoparticles on a solid support. Electrophoretic deposition (EPD) is considered a suitable technique for the fabrication of devices with biotechnological applications.11 In traditional EPD, a dc voltage is applied across the cell, thereby creating an electric field that transports charged particles to the electrodes where they deposit to form a cast film. The thickness and structure of coatings obtained by EPD can be easily adjusted by the processing parameters like deposition time, electric field and concentration of the suspension.11–13
Experimental
Reagents and materials
Four different polymeric particles were kindly supplied by NanoMyP® (http://www.nanomyp.com). PolymP®-H are polymeric particles functionalized with carboxyl groups, PolymP®-Cl are functionalized with chloride groups, PolymP®-Epoxy are functionalized with epoxy groups and PolymP®-Link are functionalized with a preactivated surface containing vinyl groups.For the immobilization of glucose oxidase on their surface, glucose oxidase (GOx) from Aspergillus niger (EC 1.1.3.4), N-ethyl-N′-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC), N-hydroxysuccinimide (NHS), potassium dihydrogen phosphate (KH2PO4), potassium monohydrogen phosphate (K2HPO4) and N,N-dimethylformamide (DMF) were all purchased from Sigma Aldrich.
To test the activity of GOx, horseradish peroxidase (HRP) from horseradish (E.C.1.11.1.7), D-glucose, 4-aminoantipyrine (4-AAP) and phenol were purchased from Sigma Aldrich.
For the synthesis of the oxygen sensitive particles, the following chemicals were used: poly(styrene-co-maleic anhydride) polymer (PSMA, 7% maleic anhydride, Mw = 224![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1) and tetrahydrofuran (THF) from Sigma Aldrich, and Pt(II) meso-tetra(pentafluorophenyl)porphine (PtTFPP) from Frontier Scientific.
000 g mol−1) and tetrahydrofuran (THF) from Sigma Aldrich, and Pt(II) meso-tetra(pentafluorophenyl)porphine (PtTFPP) from Frontier Scientific.
All chemicals were of analytical grade and used without further purification. All the solutions were prepared using doubly distilled water.
Apparatus and measurements
The UV-visible absorption spectra were recorded on a Varian Cary 50 UV-Vis spectrophotometer. All luminescence measurements were carried out on a Varian Cary-Eclipse fluorescence spectrophotometer.The average size of the particles, size distribution and zeta potential were determined with a particle size analyser Zetananosizer (Malvern Instrument, model Zetasizer Nano ZS, http://www.malvern.com).
The pH of the buffer solutions was controlled using a digital pH meter (Crison micropH 2000) calibrated at 20 ± 2 °C.
Immobilization of the enzyme
The enzyme was immobilized on four different polymeric particles involving several covalent immobilization methods.3,4 PolymP®-H (functionalized with carboxyl groups), PolymP®-Cl (functionalized with chloride groups), PolymP®-Epoxy (functionalized with epoxy groups) and PolymP®-Link (functionalized with activated vinyl groups) were used as supports. The principle of coupling the enzyme with each support surface is illustrated in Fig. 1. The following variables were optimized for all the immobilizations: temperature, enzyme concentration, reaction time and pH.In detail, immobilization on PolymP®-Cl and PolymP®-Epoxy particles was carried out by incubating 100 μL of 10 mg mL−1 enzyme solution in 100 mM potassium phosphate buffer at pH 8 containing 1.2 mL of particle suspension (0.17% w/v H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH (5
MeOH (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)). This amount corresponds to 2 mg of particles instead of 5 mg used for the rest of particles. It is due to the lack of dispersion of these kinds of particles in the immobilization media.
1)). This amount corresponds to 2 mg of particles instead of 5 mg used for the rest of particles. It is due to the lack of dispersion of these kinds of particles in the immobilization media.
Immobilization on PolymP®-Link particles was carried out by incubating 400 μL of 2.5 mg mL−1 enzyme solution in 100 mM potassium phosphate buffer at pH 8, in 100 μL of particle suspension (5% w/v); 5 mg of particles.
For the immobilization on PolymP®-H, the carboxylic acid groups of the particles were first pre-activated.4,14 Briefly, 100 μL of particle suspension (5% w/v; equivalent to 5 mg of particles) were immersed in 400 μL of DMF containing 3.1 mg EDC and 46 mg NHS for 1 h. After that, the particles were washed with phosphate buffer solution (100 mM, pH 8.0) three times, and transferred to 400 μL of the same buffer solution containing 2.5 mg mL−1 of enzyme. The immobilization was allowed to proceed at room temperature for 20 min under rotational stirring.
Thereafter, all immobilized derivatives were separated from the solution by centrifugation, decantation, and washing with fresh buffer solution and with ionic strength (NaCl 1 M) until no enzyme signal was detected in the washing solvent. The separated particles loaded with immobilized enzymes were resuspended in 0.5 mL of phosphate buffer at pH = 8 and stored at 4 °C. The collected supernatants were preserved for further testing.
All the experiments were replicated three times in order to evaluate the error  , where s is the standard deviation, t the student t and n the number of replicas.
, where s is the standard deviation, t the student t and n the number of replicas.
Characterization of functionalized particles
The amount of enzyme immobilized on particles was calculated by measuring the concentration of the enzyme in the supernatant and then subtracting from the total free enzyme amount, assuming that the loss of enzyme in each experiment could be negligible. The enzyme concentration in the supernatant solutions was measured by the spectrophotometric method at 348 nm.15 For each sample, the experiments were replicated three times to estimate the error.The relative activity (%) of the immobilized enzyme was determined using the colorimetric method based on Trinder's reaction.16–18 GOx catalyses the oxidation of D-glucose to gluconic acid with the concurrent release of hydrogen peroxide. In the presence of peroxidase (horseradish peroxidase (HRP)), the produced hydrogen peroxide reacts with 4-aminoantipyrene (4-AAP) and phenol to form a red coloured quinoneimine dye, which has an absorption maximum at 510 nm. Based on this, the relative activity was calculated as a percentage of the immobilized GOx and free GOx absorbances.
In detail, to test the relative activity (%) of the immobilized GOx, 2.5 mL phosphate buffer (100 mM, pH 8.0) containing 2 μg free or immobilized GOx and 0.04 mmol glucose were mixed with 1 mL of 4-aminoantipyrine (200 mM), 1 mL of phenol (200 mM), and 0.5 mL HRP (20 U mL−1) and incubated at 37 °C and stirred for 30 min. Afterward, the testing solution was analysed using a UV-Vis spectrophotometer at a wavelength of 510 nm.18
Preparation and characterization of the oxygen sensitive particles
The PtTFPP–PSMA particles were prepared following the procedure described elsewhere.11,19 Briefly, 100 mg of PSMA (0.1% w/v) and 1.5 mg of PtTFPP (1.5% w/w respect to the polymer) were dissolved in 100 mL of THF. Then, 5 mL of the cocktail were added dropwise, with stirring, to 8 mL of deionized water. Finally, THF was evaporated under a stream of air.The resulting polymeric particles with the embedded dye formed stable dispersions and presented an average particle diameter of 151.3 nm, a PdI of 0.088, a zeta potential of −32.9 mV and a conductivity of 0.0330 mS cm−1.
Electrophoretic deposition
For the preparation of the biosensing film, oxygen sensitive particles and GOx functionalized particles were both deposited on the surface of a gold chip by EPD. The homemade electrophoretic cell previously described by our research group11 was used to carry out the electrophoretic deposition and several deposition strategies were tested. The chips were rinsed with deionized water and dried with nitrogen to remove all non-attached particles after each deposition.To improve the adherence to the substrate material and to increase the density of the coating, different potentials and deposition times were also tested.
Results and discussion
Determination of optimal conditions for enzyme immobilization
Four different methods for the covalent immobilization of enzymes were evaluated using GOx as a model enzyme. Two essential requirements must be met to achieve efficient covalent binding between the enzyme and the carrier: a suitable reactivity of the functional groups of the selected carrier and sterically accessible functional groups to each other under the selected immobilization conditions before binding. Based on this, GOx was directly covalently immobilized on PolymP®-Cl (functionalized with chloride groups), PolymP®-Epoxy (functionalized with epoxy groups) and PolymP®-Link (functionalized with a pre-activated surface containing vinyl groups). These functional groups may be used for covalent immobilization without any previous surface activation treatment. Whereas, the binding of the enzyme to PolymP®-H (functionalized with carboxyl groups) was carried out by initial activation of the surface using carbodiimide, followed by enzyme coupling to the activated support.20 The mechanisms of coupling the enzyme with these support surfaces are presented in Fig. 1.Optimization of the immobilization of the enzyme on carboxyl functionalised particles
The first step was to optimise the activation of the carboxyl groups on PolymP®-H particles. To do this, the amount of EDC, the amount of NHS and the reaction time were evaluated. Table 1 shows the optimal conditions for activating the carboxyl groups.| Particle | Activation | GOx immobilization | |||
|---|---|---|---|---|---|
| [GOx] (mg mL−1) | Media | Time (min) | Amount of GOx per mass of particles (mg g−1) | ||
| PolymP®-H | [EDC] = 40 mM, [NHS] = 0.8 M in DMF for 1 h | 1.0 | 100 mM phosphate buffer pH 8 | 20 | 51 |
| PolymP®-Link | Not needed | 20 | 12 | ||
| PolymP®-Cl | Not needed | 30 | 35 | ||
| PolymP®-Epoxy | Not needed | 60 | 45 | ||
To investigate the optimum pH for immobilizing GOx, 5 mg of activated carboxylated particles were added to 400 μL of 2.5 mg mL−1 GOx prepared in 100 mM buffer solution at different pH values (6, 7, 8 and 9). The immobilization mixture was kept at room temperature for one hour and then was washed with the same buffer solution until no protein was detected in the washing solvent. As can be seen in Fig. 2a, the pH of the immobilization medium significantly affects both the amount of immobilization and the activity. The maximum amount of immobilized enzyme (0.051 mg enzyme per mg support) and the maximum relative activity (%) were both found at pH 8.0. Therefore, it was selected as the optimum pH for further experiments.
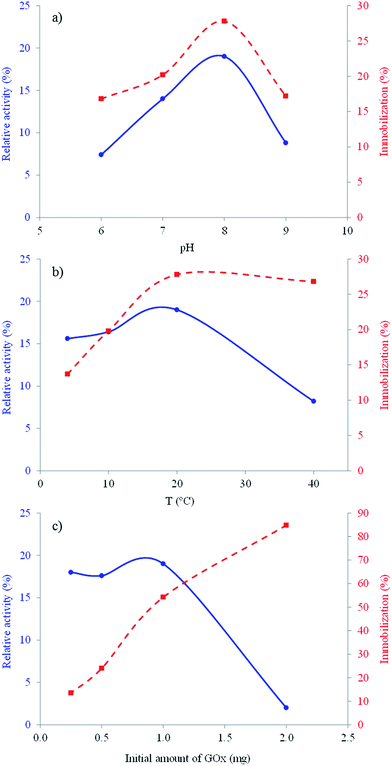 | ||
Fig. 2 Effect of the (a) pH, (b) temperature and (c) initial amount of enzyme versus the amount of immobilized enzyme ( ) and its relative activity (%) after immobilization ( ) and its relative activity (%) after immobilization ( ). ). | ||
To determine the effect of the temperature, immobilization was carried out at different temperatures (4, 10, 20 and 40 °C). Fig. 2b shows the experimental results. It is known that temperature is an important parameter which determines the three-dimensional structures or conformational states of enzymes, and therefore, as expected, it affects both immobilization and activity.21,22 The amount of immobilized enzyme increases with the temperature, while the relative activity (%) drops significantly. Based on this result, the temperature selected for immobilizing GOx was 20 °C. Under these conditions, the immobilized enzyme retained approximately 20% of the native enzymatic activity.
The effect of the enzyme concentration was also investigated. GOx solutions with four different concentrations (0.625, 1.25, 2.5 and 5 mg mL−1) were prepared with a buffer solution at pH 8.0. Then, 400 μL of each solution was added onto 5 mg of activated particles for 20 min at 20 °C. Fig. 2c shows that the amount of immobilized enzyme increases when the initial amount of enzyme is increased. However, there is a pronounced decrease in the relative activity of the immobilized enzyme when the initial concentration amount of GOx is higher than 1 mg mL−1. This phenomenon could be attributed to the aggregation of the enzyme on the surface of particles that might cause the blockage of enzyme active sites, resulting in a drop in immobilized enzyme activity. These results clearly showed that a higher amount of immobilization of GOx on the support does not mean that it will exhibit higher activity. These effects were previously observed in the literature.23
Table 1 shows an overview of the optimal conditions for effective immobilization of GOx on PolymP®-H attending not only to the amount of immobilized enzyme but also to its activity after immobilization.
Optimization of immobilization of enzyme on chloride, epoxy and active vinyl functionalised particles
Having the optimal conditions for immobilizing the enzyme into PolymP®-H as a starting point, pH, incubation temperature and concentration of the enzyme were also evaluated for the rest of polymeric particles (PolymP®-Cl, PolymP®-Epoxy and PolymP®-Link). In this case, the immobilization of the enzyme was carried out directly; no preactivation stage was necessary. Similar results were obtained for PolymP®-H. The results have been summarized in Table 1.Comparison of the immobilizing behaviours using different functional groups
To compare the immobilizing behaviours on different supports with different superficial chemistry, we investigated the variation of the immobilized amount of the enzyme and its activity under the optimum conditions versus the time between 20 min and 48 h (see Fig. 3). | ||
| Fig. 3 Change of the (a) amount of immobilized enzyme and (b) the relative activity (%) versus the incubation time at 37 °C. | ||
An increase in the incubation time provides an increase of the amount of immobilized enzyme for all the particles, but this increase is more effective for PolymP®-Link and PolymP®-H than for the others (see Fig. 3a). Thus, it is possible to immobilize more amounts of enzyme in a shorter time using PolymP®-Link and PolymP®-H.
Fig. 3b shows that the relative activity (%) of the immobilized enzyme is decreased when the incubation time increases, but the highest activity is exhibited by the enzyme immobilized on PolymP®-Link particles at all the incubation times followed by carboxylic PolymP®-H particles. The relative activity (%) of the enzyme immobilized on PolymP®-Link was 26% of the free enzyme while it was 19% when it was immobilized on PolymP®-H for 30 min.
It is possible to conclude that PolymP®-Link particles show a similar or better superficial chemistry for the immobilization of enzymes than classical particles based on carboxyl groups. In addition, Table 1 summarises the amount of enzyme immobilised per mass of particles under the optimum conditions. It is possible to conclude that PolymP®-Link need the lowest amount of enzyme (only 12 mg per gram of particles) for providing the highest activity. Thus, its use provides a considerable reduction in the cost of the final biosensor.
The active vinyl functionalised particles show the best results even when they have the lowest amount of immobilized enzyme because the active centre of the biomolecule after immobilisation in PolymP®-Link might be more available in this kind of particle than in the rest of the tested supports. Or it might be due to a better stabilization of the quaternary structure of GOX after attachment to an active vinyl group than to any of the other ones.
Optimization of the EPD experimental conditions and applications
Different approaches were accessed for depositing both oxygen sensitive and enzyme-functionalized particles. However, deposition of both at the same time did not provide good results, probably due to the difference of charge between them. Therefore, deposition protocols consisting of alternating layers of oxygen sensitive and GOx-functionalized particles were evaluated. The optimal response was found by depositing a layer of oxygen sensitive particles first and then a layer of enzyme-functionalized particles.The electrodeposition of oxygen sensitive particles was evaluated by applying constant potentials (2, 4 and 6 V cm−1) and deposition times between 10 and 120 min, at a constant concentration of suspended particles and measuring the differences in the luminescence intensity (Ix − I0), where Ix corresponds to the relative luminescence intensity at 8 ppm of oxygen and I0 is the relative luminescence intensity in the absence of oxygen. ESI shows the experimental results (Fig. ESI-1).†
The response signal (Ix − I0) is proportional to the amount of deposited particles therefore it is possible to conclude that an increase of the deposition time provides an increase on the amount of deposited particle up to a certain deposition time in which the amount of particles remains constant. This is expected in a constant voltage EPD because at the initial period of EPD there is generally a linear relationship between deposition mass and time during12,24 and when the deposition time increases, the electric field influencing electrophoresis decreases because of the formation of an insulating layer of deposited particles on the electrode surface. The maximum response to the oxygen concentration was achieved at 6 V cm−1 and a deposition time of 30 min.
In addition, luminescence spectra were recorded to evaluate possible changes in the spectroscopic behaviour of the oxygen sensitive particles once the particles were deposited. No changes in the spectroscopic properties of deposited particles compared to particles in suspension were observed.
The effect of applied potential and deposition time on the electrophoretic deposition of immobilized GOx was evaluated by measuring the sensing response to 100 mg mL−1 glucose when constant potentials between 2 and 8 V cm−1 and the deposition time between 10 and 60 min were applied at a constant concentration of particles. ESI shows the experimental results (Fig. ESI-2).† Deposition times longer than 50 min and potentials higher than 6 V cm−1 led to the darkening of the deposit. Moreover, high potentials caused electrolysis of water and evolution of hydrogen and oxygen gases at the electrodes on application of an electric field. The incorporation of these gas bubbles in the deposit led to decrease the quality of the coating. The optimal parameters were found to be potential, 6 V cm−1 and deposition time, 30 min.
In addition, the number of alternating layers was also tested but the response was the same as for 1 layer of each kind of particle.
In order to determine the applicability of each functional group under study in the field of biosensing, the response of each chips was evaluated with different glucose concentrations (2, 5 and 10 mg mL−1), in 100 mM phosphate buffer solution at pH 7.0. The activity of the enzyme remained constant during all the measurement and no dye photobleaching or leaching of the particles from the chip surface was detected. Fig. 4 and ESI (Fig. ESI-3†) show the experimental results. They show that GOx immobilized on PolymP®-Link provided the best response, which was 50 times higher than the response showed by the classical carboxylated particles.
 | ||
| Fig. 4 Responses of the four glucose sensing films at a concentration level of 2 mg mL−1. Detector voltage 600 V, λexc/em = 395/650 nm, monochromator slit widthexc/em = 20/20 nm. | ||
Conclusions
Four different types of polymeric particles with different superficial chemistry have been tested with the aim to determine the effect of the superficial functional group on chemical immobilization of the enzyme in the field of optical biosensing, using GOx as a model enzyme. In fact the immobilization of the enzyme has been optimised on chloride-functionalized (PolymP®-Cl), epoxy-functionalized (PolymP®-Epoxy), preactivated surface containing vinyl groups (PolymP®-Link) and classical carboxyl-functionalized (PolymP®-H) particles; PolymP®-H were also used as a reference material.The results showed that the enzyme immobilization is hardly influenced by the chemistry of the carrier, pH, temperature, enzyme concentration, and immobilization time. Combining the results of activity and enzyme immobilization, it was determined that the particle with a preactivated surface containing vinyl groups (PolymP®-Link) provides higher activity with a smaller amount of enzyme than the classical carboxyl-functionalized (PolymP®-H) particles, which are widely used for immobilizing biomolecules.
To demonstrate their applicability in the field of biosensing, the four enzyme-functionalized particles were deposited with oxygen sensitive particles in gold chips using EPD. The chip obtained using enzyme-functionalized PolymP®-Link showed the highest sensitivity to glucose, providing a sensing response 50 times higher than when classical carboxyl functionalized particles were used.
It is possible to conclude that particles with a preactivated surface containing vinyl groups (PolymP®-Link) are a serious alternative to classical polymeric particles for effective immobilisation of biomolecules in the development of biosensing films.
Other interesting parameters to be considered are kinetic parameters (Km and Vmax) and the reusability and stability of immobilized enzymes. In this work they have not been included because these are not only related to the functional groups but also with the selected biomolecule. Thus, they should be evaluated when the aim of the research is the development of a sensor.
Acknowledgements
The authors gratefully acknowledge the financial support of the Spanish Ministry of Economy and Competitiveness (CTQ2011-25316, Ramon-Marquez's grant reference AP2012-0944 and Medina-Castillo's Torres Quevedo contract reference PTQ-11-04904) and the People Programme (Marie Curie Actions, Multi-ITN) of the European Union’s Seventh Framework Programme (project EUROMBR grant agreement no. 608104).References
- M.-S. Steiner, A. Duerkop and O. S. Wolfbeis, Chem. Soc. Rev., 2011, 40, 4805–4839 RSC.
- H. Susanto, A. M. Samsudin, N. Rokhati and I. N. Widiasa, Enzyme Microb. Technol., 2013, 52, 386–392 CrossRef CAS PubMed.
- Carrier-bound Immobilized Enzymes: Principles, Applications and Design, ed. L. Cao, WILEY-VCH, 2006 Search PubMed.
- Bioconjugate Techniques, ed. G. T. Hermanson, Academic Press, 2008 Search PubMed.
- Y. Amao, T. Miyashita and I. Okura, J. Fluorine Chem., 2001, 107, 101–106 CrossRef CAS.
- S. Medina-Rodríguez, A. de la Torre-Vega, J. F. Fernández-Sánchez and A. Fernández-Gutiérrez, Sens. Actuators, B, 2013, 176, 1110–1120 CrossRef PubMed.
- W. Trettnak, C. Kolle, F. Reininger, C. Dolezal and P. O'Leary, Sens. Actuators, B, 1996, 36, 506–512 CrossRef CAS.
- Y. Amao, Microchim. Acta, 2003, 143, 1–12 CrossRef CAS.
- X.-d. Wang and O. S. Wolfbeis, Chem. Soc. Rev., 2014, 43, 3666–3761 RSC.
- G. Mistlberger, K. Koren, E. Scheucher, D. Aigner, S. M. Borisov, A. Zankel, P. Pölt and I. Klimant, Adv. Funct. Mater., 2010, 20, 1842–1851 CrossRef CAS.
- M. Marin-Suarez, S. Medina-Rodriguez, O. Ergeneman, S. Pane, J. F. Fernandez-Sanchez, B. J. Nelson and A. Fernandez-Gutierrez, Nanoscale, 2014, 6, 263–271 RSC.
- Electrophoretic Deposition of Nanomaterials, ed. J. H. Dickerson and A. R. Boccaccini, 2012 Search PubMed.
- S. Seuss and A. R. Boccaccini, Biomacromolecules, 2013, 14, 3355–3369 CrossRef CAS PubMed.
- Y. Gao and I. Kyratzis, Bioconjugate Chem., 2008, 19, 1945–1950 CrossRef CAS PubMed.
- Q. Shao, P. Wu, X. Xu, H. Zhang and C. Cai, Phys. Chem. Chem. Phys., 2012, 14, 9076–9085 RSC.
- J. A. Lott and K. Turner, Clin. Chem., 1975, 21, 1754–1760 CAS.
- S. Rauf, A. Ihsan, K. Akhtar, M. A. Ghauri, M. Rahman, M. A. Anwar and A. M. Khalid, J. Biotechnol., 2006, 121, 351–360 CrossRef CAS PubMed.
- X. Hou, B. Liu, X. Deng, B. Zhang, H. Chen and R. Luo, Anal. Biochem., 2007, 368, 100–110 CrossRef CAS PubMed.
- S. M. Borisov, T. Mayr, G. n. Mistlberger, K. Waich, K. Koren, P. Chojnacki and I. Klimant, Talanta, 2009, 79, 1322–1330 CrossRef CAS PubMed.
- A. Sassolas, L. J. Blum and B. D. Leca-Bouvier, Biotechnol. Adv., 2012, 30, 489–511 CrossRef CAS PubMed.
- M. Y. Arica and V. Hasirci, J. Chem. Technol. Biotechnol., 1993, 58, 287–292 CrossRef CAS.
- V. Bulmuş, H. Ayhan and E. Pişkin, Chem. Eng. J. Biochem. Eng. J., 1997, 65, 71–76 CrossRef.
- P. Ye, R. B. Wan and X. P. Wang, J. Mol. Catal. B: Enzym., 2009, 61, 296–302 CrossRef CAS PubMed.
- I. Zhitomirsky and L. Gal-Or, J. Mater. Sci.: Mater. Med., 1997, 8, 213–219 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ay00103j |
| This journal is © The Royal Society of Chemistry 2015 |