Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The application of high resolution diffusion NMR for the characterisation and quantification of small molecules in saliva/dentifrice slurries
Adam
Le Gresley
*a,
Emma
Simpson
a,
Alex J.
Sinclair
a,
Neil
Williams
a,
Gary R.
Burnett
b,
Dave J.
Bradshaw
b and
Robert A.
Lucas
b
aSEC Faculty Kingston University, Kingston University, Kingston upon Thames, KT1 2EE, UK. E-mail: a.legresley@kingston.ac.uk; Fax: +44 (0)208 4177497; Tel: +44 (0)208 4177432
bGlaxoSmithKline Consumer Healthcare, Weybridge, KT13 0DE, UK
First published on 14th January 2015
Abstract
The application of DOSY (Diffusion Ordered Spectroscopy) NMR as a technique for the virtual separation of toothpaste adjuvants in model saliva is reported for the first time. In addition, the scope and limitations of DOSY NMR are considered using the DOSY Tool Box processing software, as is the quantification of the adjuvants and components of saliva by quantitative NMR (qNMR). These techniques represent a new and powerful tool for the evaluation of complex mixtures of natural products with a view to identifying biomarkers for disease within the oral cavity.
1. Introduction
Within the field of oral care research Nuclear Magnetic Resonance (NMR) techniques have barely been considered as a technique for characterising the small molecule components of saliva or those anti-microbial adjuvants in a dentifrice slurry. The main references to NMR in the oral sciences focus on solid state (MAS) 19F and 31P NMR spectroscopy and the potential application of advanced 2D and pseudo 2D NMR techniques have largely been ignored.1The direct application of Diffusion Ordered Spectroscopy (DOSY) NMR and quantitative NMR (qNMR) for the characterisation and quantification of small molecules, natural products and biomarkers in saliva/dentifrice slurry models has until now gone unreported.
To augment the effectiveness of toothpaste a number of actives are added to improve the health of both hard and soft tissues found in the oral cavity. These fall into three broad categories: anti-caries actives (e.g. sodium fluoride, sodium monofluorophosphate and amine fluoride), sensitivity actives (e.g. potassium nitrate, potassium chloride, strontium acetate, stannous fluoride and bioactive glass), gum health actives, comprising mainly antimicrobial agents such as triclosan, zinc citrate, stannous, essential oils, isopropylmethylphenol plus a wide range of other natural products.2–5 Despite a considerable number of publications on dental plaque pathogenicity and biofilm formation, there are relatively few specific studies looking at the biological mode of action/activity of natural products.6,7 Many of the actives evaluated are well-known secondary plant metabolites of the polyphenol class; however, the majority of agents are non-specific anti-microbial agents, having an impact on a variety of oral microorganisms in a range of ways. The antimicrobial effects of such agents are generally measured as the minimum bactericidal concentration (MBC) which kills particular microbes, or the minimum inhibitory concentration (MIC) which prevents bacterial growth. However, in a flow through environment such as the mouth. the longevity and retentivity in the oral cavity (often termed substantivity) of agents are also crucial to their efficacy. For example during toothbrushing, antimicrobial agents may be present at greater than the MBC or MIC for short periods. However, at sub-MIC concentrations, agents may have a range of more subtle, but still important effects, such as reducing microbial (re)growth, inhibiting key metabolic processes such as acid production, protease activities or polysaccharide synthesis interfering with bacterial adhesion.8,9 Understanding where agents reside in the oral cavity, for how long, and to which components they bind are therefore crucial to improving oral care product efficacy. Such understanding could then lead to more rational approaches to optimising product formulation and active delivery. The reported difficulties when attempting to evaluate the efficacy of a natural product are predicated on the complexity of the often crude mixtures of natural products available and the technical challenges of time-consuming purification steps.10 The importance of basic chemical characterisation of small/natural extract molecules cannot be overstated, particularly where the synergistic effects of more than one component are being investigated.6
Recent improvements in high resolution NMR instrumentation, coupled with the exceptional work conducted in the improvement of flexible processing software has made DOSY NMR an increasingly valuable tool in complex mixture analysis,11–13 including biofluids and drug preparations, simultaneously identifying and in part quantifying their components.14–16 The variation in isolation and derivatisation methods has led to often inconsistent data and, despite the excellent review by Koo et al.6 there is clearly scope for the evaluation of DOSY NMR as a non-destructive analytical method, which requires little standardisation. The NMR experiments are efficient, with data being acquired in just over one hour from sample collection. This, coupled with the ability to discriminate NMR signals on the basis of size (hydrodynamic radius), can obviate the need for 1D NMR spectral “binning” of multiple chemical shift regions when attempting to identify principal components. Through internal standardisation, approximate data regarding the mass of a component can be achieved. The aims of this communication are primarily to consider the scope and limitation of DOSY NMR as tools for the characterisation of natural product adjuvants in saliva models and saliva/dentifrice slurries and to demonstrate the potential for internally standardised qNMR to be used to quantify individual component concentrations, without the need for protracted separation and purification steps. For the purpose of evaluating the application of DOSY NMR/qNMR the organic antimicrobial preservative 4-isopropyl-3-methyl phenol (IPMP) was initially used as a reference compound in different matrices: these included a simple surfactant solution, model saliva and saliva/dentifrice slurry.
Whilst MS methods have been used to analyse complex mixtures, the sample preparation and purification processes influence how the molecules of interest interact with other components in solution.17 MSn methods have been shown to be useful in these scenarios but the technique is still destructive. Use of conventional 2D techniques for molecular identification have been used to verify ambiguous signals and should be used in tandem with a technique such as DOSY. Whilst it is possible to use for example HSQC to separate 1H overlaps and aid in signal assignment, the possible interactions between the various components meant we were just as interested in characterising their environment as much as identifying components individual molecules. This will prove of particular value for our current research, which looks at the interactions of such molecules with proteins found in saliva in gingivitis. All of the above mentioned techniques were used in the cases of ambiguity. The interaction between SDS and the IPMP, whilst predictable, illustrates the merit of this technique in a simple system such as that discussed. The application of DOSY to identify these environments and also individual molecules has been previously reported for other complex mixtures and this work aimed to extend this methodology further.
2. Materials and methods
Being phenolic and having similar chemical and physical motifs to other naturally occurring phenolic compounds, IPMP provided a convenient model for DOSY NMR/qNMR method development. Using IPMP, DOSY NMR was used to observe the influence of pH, sodium dodecyl sulphate (SDS) and salt concentration on the diffusion characteristics of different adjuvants and also their impact on the accuracy of the qNMR data at different concentrations of IPMP. It is worthy of note that the proportional change in diffusion for TSP was not significantly greater than the observed change in diffusion for other internal standards owing to increased viscosity of saliva. Interactions with proteins seem fast on the NMR timescale and therefore not particularly strong. We acknowledge, however, that this may be a limitation in this study.2.1. Simple surfactant solution
Unless otherwise stated, all chemicals were obtained from Sigma-Aldrich. Measurement of solutes was carried out on a Fisherbrand MH-214 balance. PBS Buffer solution was prepared (100 mM NaCl) and corrected to pH 6, 7, 8 & 9. The SDS was added to a concentration of 0.75% w/w and IPMP was dissolved directly in the buffer and made up to the concentration range 0.005%, 0.01%, 0.025%, 0.05% and 0.1% w/w. Each of these sample concentrations was prepared separately five times and 3 qNMR experiments run on each to validate the precision of concentration calculations. Deuterated sodium trimethylsilylpropionate (TSP) was used as an internal reference standard at 14.4 mM (DOSY) and 7.4 mM (qNMR) concentrations.2.2. Model saliva
Fresh model saliva solutions were prepared using the recipe as stated by Klimek et al.18 and used on the same day of preparation.18 The solutions were kept at 25 °C and out of direct sunlight.2.3. Model saliva/dentifrice slurry
The slurry was prepared by stirring 5 g of Aquafresh Ultimate Toothpaste (GSK, Brentford, UK) slurried in a solution of model saliva (8 ml) prepared as per Section 2.2. Five samples were prepared at each concentration (0.005%, 0.01%, 0.025%, 0.05% and 0.1% w/w wrt IPMP). The slurry was centrifuged at 4500 rpm (3089 grams) for 30 minutes to remove the solid-components of toothpaste such as silicas and titanium dioxide, and the supernatants only used for analysis. NMR experiments were undertaken in triplicate for qNMR on the supernatant.2.4. Qualitative/DOSY NMR analysis
A Bruker Avance III 600 MHz NMR spectrometer with 5 mm TXI Probe and temperature control unit was used for all 1H NMR experiments. 5 mm Bruker Single Use NMR tubes (Product Code Z117777) were used. All spectra were acquired on Topspin 3.0 (Bruker, Germany) and 64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 complex data points were acquired over a sweep width of 10.3112 ppm using a stimulated echo bipolar pulsed field gradient with 1 spoil gradient and 3-9-19 WATERGATE sequence (STEBPGP1S19). This was used to obtain the diffusion series with δ = 2.4 ms and Δ = 100 ms. The relaxation delay was set to 7 s and the WATERGATE pulse duration was 1000 μs with 64 linear gradient steps, from 2–95% gradient intensity, each consisting of 16 scans.
000 complex data points were acquired over a sweep width of 10.3112 ppm using a stimulated echo bipolar pulsed field gradient with 1 spoil gradient and 3-9-19 WATERGATE sequence (STEBPGP1S19). This was used to obtain the diffusion series with δ = 2.4 ms and Δ = 100 ms. The relaxation delay was set to 7 s and the WATERGATE pulse duration was 1000 μs with 64 linear gradient steps, from 2–95% gradient intensity, each consisting of 16 scans.
Each sample was allowed to equilibrate within the NMR spectrometer for 5 minutes. All NMR experiments were carried out at 25 °C. A sine bell shaped window function phase was applied over all data points prior to Fourier transformation (16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 384 points) using Topspin 3.0 (Bruker, Germany). Diffusion data were processed using DOSY Toolbox, (Mathias Nilsson, Manchester University) and TSP was used for Lorentzian reference deconvolution. Individual peaks were fitted exponentially after a 2nd order polynomial baseline correction was employed.15 Errors in diffusion coefficient were calculated based on the standard deviation for each diffusion curve and are in line with the estimated error as reported for a similar mixture of ca. 0.1 × 10−10 m2 s−1.19 The residual sum of squares for each of the diffusion curves was less than 5 × 10−3 in all cases.
384 points) using Topspin 3.0 (Bruker, Germany). Diffusion data were processed using DOSY Toolbox, (Mathias Nilsson, Manchester University) and TSP was used for Lorentzian reference deconvolution. Individual peaks were fitted exponentially after a 2nd order polynomial baseline correction was employed.15 Errors in diffusion coefficient were calculated based on the standard deviation for each diffusion curve and are in line with the estimated error as reported for a similar mixture of ca. 0.1 × 10−10 m2 s−1.19 The residual sum of squares for each of the diffusion curves was less than 5 × 10−3 in all cases.
1H–1H correlation spectroscopy (COSY) was used in addition to predicted Mr data from the diffusion correlation to correctly assign the 1H signals relating to the mixture of components for the commercial toothpaste/artificial saliva samples.
A 2D homonuclear shift correlation pulse sequence using gradient pulses for selection and multiple quantum filtering was used (cosygpmfqf). Size of F1 FID was 2048 with a sweep width of 7.7692 ppm and a dwell time of 161 μs and relaxation delay of 1.861 s to give an acquisition time of 0.3297 s. A sine bell shaped window function was applied over all data points prior to Fourier transformation, phasing and baseline correction using Topspin 3.0 (Bruker, Germany).
Matrix matched samples were spiked with reference standards to confirm the identity of the components where there was ambiguity. All reference standards were obtained from Sigma-Aldrich Ltd and used without further purification.
2.5. Quantitative NMR (qNMR)
The quantification of components in natural product mixtures through comparison with the internal standard TSP has already been reported.10,11 A Bruker Avance III 600 MHz NMR spectrometer with 5 mm TXI Probe and temperature control unit was used for all experiments. 5 mm Single Use NMR tubes (Bruker, Product Code Z117777) were used. All spectra were acquired on Topspin 3.0 and 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 536 complex data points were acquired over a sweep width of 12.9909 ppm using a 90° pulse angle and an acquisition time of 4.2030 s. A sine bell shaped window function phase shifted by 90° was applied over all data points prior to Fourier transformation, phasing and baseline correction using Topspin 3.0. The chemical shift of all data was referenced to the TSP reference 0 ppm. All spectra were acquired at 25 °C. Three replicates of the qNMR experiments described were carried out for each of the five IPMP samples and the 5 different concentrations. The average integral of all IPMP signals was used for quantification and all IPMP signals had a s/n ratio greater than 200 making them acceptable for qNMR processing. Quantitative data obtained using this method has been shown to compare well to traditional LC-MS and LC-UV techniques.20
536 complex data points were acquired over a sweep width of 12.9909 ppm using a 90° pulse angle and an acquisition time of 4.2030 s. A sine bell shaped window function phase shifted by 90° was applied over all data points prior to Fourier transformation, phasing and baseline correction using Topspin 3.0. The chemical shift of all data was referenced to the TSP reference 0 ppm. All spectra were acquired at 25 °C. Three replicates of the qNMR experiments described were carried out for each of the five IPMP samples and the 5 different concentrations. The average integral of all IPMP signals was used for quantification and all IPMP signals had a s/n ratio greater than 200 making them acceptable for qNMR processing. Quantitative data obtained using this method has been shown to compare well to traditional LC-MS and LC-UV techniques.20
3. Results
3.1. DOSY NMR
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D = a D = a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mr + b. Mr + b. | (1) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D and Mr for IPMP in simple solution (r2 = 1) values for a and b constants of −0.37 and + 1.49 respectively. The value of a agrees well with the literature value but the value of b is substantially lower owing to the relatively high viscosity of water when compared to common organic solvents. This has been reported previously by our group.10
D and Mr for IPMP in simple solution (r2 = 1) values for a and b constants of −0.37 and + 1.49 respectively. The value of a agrees well with the literature value but the value of b is substantially lower owing to the relatively high viscosity of water when compared to common organic solvents. This has been reported previously by our group.10
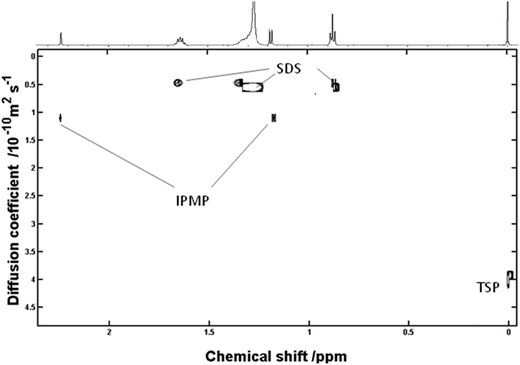
Fig. 1 shows a generally good correlation of mass vs. diffusion for all components with the exception of IPMP in the presence of sodium dodecyl sulphate (SDS). As reported by Nilsson et al.23 association of lipophilic molecules to SDS micelles substantially decreases the apparent diffusion of these molecules and can actually be used to resolve compounds of similar mass in carefully controlled matrix assisted diffusion experiments. The deviation from linearity indicated the association of IPMP with a molecule/aggregate of substantially higher Mr.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mr and log
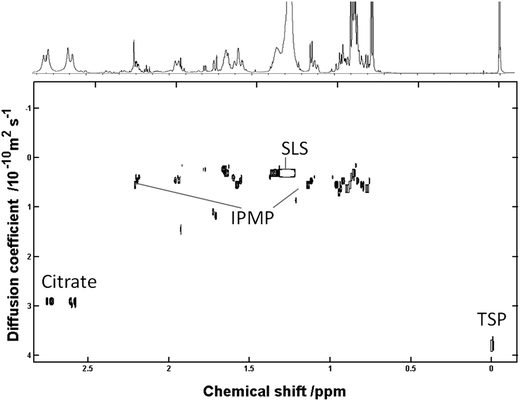
Mr and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D for different systems, samples of commercial toothpaste (n = 3) were analysed to validate the capacity of DOSY NMR for virtual separation of components of dentifrice slurry. Fig. 4, 5 and 6 show aliphatic, carbohydrate and aromatic regions of the averaged pseudo 2D DOSY plots. By determining the constants a and b for the log
D for different systems, samples of commercial toothpaste (n = 3) were analysed to validate the capacity of DOSY NMR for virtual separation of components of dentifrice slurry. Fig. 4, 5 and 6 show aliphatic, carbohydrate and aromatic regions of the averaged pseudo 2D DOSY plots. By determining the constants a and b for the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D/log
D/log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mr correlation it was possible to directly extract approximate mass data for lipophilic components with reasonable accuracy (±4%). These predicted mass values coupled with chemical shift and coupling data from 1H and 1H 1H COSY experiments were used to assign the signals in the DOSY plots as shown.
Mr correlation it was possible to directly extract approximate mass data for lipophilic components with reasonable accuracy (±4%). These predicted mass values coupled with chemical shift and coupling data from 1H and 1H 1H COSY experiments were used to assign the signals in the DOSY plots as shown.
 | ||
| Fig. 5 Carbohydrate region of 2D DOSY plot of commercial toothpaste and artificial saliva, showing the distinct separation of glycerol from sorbitol. | ||
3.2. qNMR
Quantitative NMR (qNMR) was first described in 1963 and is increasingly regarded as a powerful non-destructive technique with traceability to SI units, providing advantages over many other analytical methods.24,25 The development of affordable high field NMR instruments coupled with more elegant pulse sequences has prompted a recent flurry of activity in the field of NMR analysis, including human and plant metabolomic studies.26,27 A recent review of the applications of qNMR over the last eight years highlights its growing importance in the context of metrology and supports the statement that qNMR can be regarded as a primary method of purity analysis for organic compounds.28 Using TSP as the internal standard, not only for diffusion but also for quantification, enables the calculation of unknown analytes by comparing the integral of known and unknown signals. Despite growing acceptance of this technique, there are acquisition and processing factors which can contribute to uncertainty when calculating concentrations of analytes. When dealing with complex mixtures with often high ionic strength (Q-factor), accuracy can be substantially reduced29 and it is important that the matrix in which the analytes appear is properly characterised. A key part of the current work is to consider the impact of high ionic strength, viscous matrices and the direct impact on the ease with which qNMR experiments can be efficiently and accurately undertaken. Fig. 7 shows the effect of pH and co-solvents/surfactants on the accuracy with which IPMP can be quantified. The impact of viscosity and high salt content from the artificial saliva on the validity of this technique to quantify IPMP is shown in Fig. 8. The direct application of qNMR in dentifrice slurry is evaluated in Fig. 9. The range of concentrations was deliberately reduced in size to highlight the limits of quantification using this method with potential applications in real-world saliva samples. The impact of pH on the correlation graphs is shown in all cases.4. Discussion
4.1. Virtual separation of the components
Whilst the virtual separation of small molecules in mixtures using DOSY NMR is established,14 we report the analysis of dentifrice slurry using DOSY NMR for the first time. Use of TSP as an internal diffusion standard enables the association of the IPMP to SDS micelles to be clearly observed (Fig. 2) and for simple systems where there is no competition it is possible to extract information as to what extent association is occurring between host and guest based on eqn (2), discussed in the review by Fielding.16| Dobs = XIDI + XISDIS | (2) |
Using the drop in diffusion coefficient for TSP upon addition of SDS as a marker of increased viscosity, it is possible to correct the unbound Diffusion coefficient and calculate the approximate mole fraction for bound and unbound fractions of IPMP. The extent to which the diffusion coefficient is reduced as a consequence of association with SDS micelles (Mr ∼ 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), suggests that 60% IPMP is bound to SDS and 40% unbound. It should be stated that the lack of additional signals for IPMP in the presence of SDS suggests fast exchange on the NMR timeframe. This finding is in good agreement with the measured exchange rates of sparingly soluble fluorescent probes in SDS micelles, which has been previously measured on the microsecond time frame.13
000), suggests that 60% IPMP is bound to SDS and 40% unbound. It should be stated that the lack of additional signals for IPMP in the presence of SDS suggests fast exchange on the NMR timeframe. This finding is in good agreement with the measured exchange rates of sparingly soluble fluorescent probes in SDS micelles, which has been previously measured on the microsecond time frame.13
Whilst the inclusion of artificial saliva does little to impact the separation of the organic components, the limitation of DOSY NMR becomes more apparent when commercial toothpaste is included. It has previously been reported10,14 that it is possible to correlate Mr with D and this appears to be independent of viscosity. From Fig. 1 it is possible to observe a trend for Citrate, TSP, and glycerol and obtain a and b constant values of −0.803 and 2.332 respectively. This enabled the prediction of Mr from log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D values and this correlation coupled with 1H 1H COSY data enabled the identification of saccharin, sorbitol and urea in the dentifrice slurry (Fig. 5). A linear trend for the log
D values and this correlation coupled with 1H 1H COSY data enabled the identification of saccharin, sorbitol and urea in the dentifrice slurry (Fig. 5). A linear trend for the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D vs. log
D vs. log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mr exists, and this can be observed in this case for the suitably hydrophilic components.
Mr exists, and this can be observed in this case for the suitably hydrophilic components.
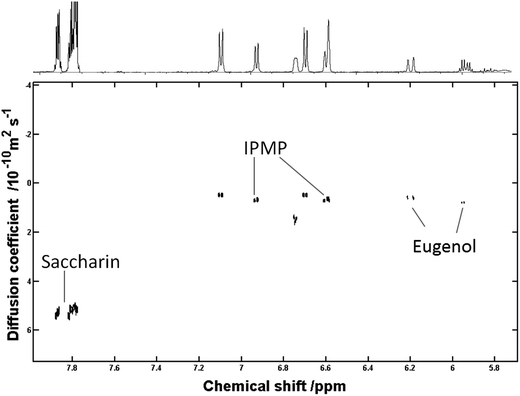
However, the lipophilic components, which are associated with SDS micelles, appear to deviate from the linear trend, rendering prediction of Mr with any accuracy difficult. The attempt to extrapolate approximate Mr values for unknown components using a log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D vs. log
D vs. log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mr would appear limited to those components that do not associate with SDS micelles. Some of the components, which appeared micellised, could be identified through 1H 1H COSY and included eugenol (Fig. 6) and (−) menthol.
Mr would appear limited to those components that do not associate with SDS micelles. Some of the components, which appeared micellised, could be identified through 1H 1H COSY and included eugenol (Fig. 6) and (−) menthol.
Recent work on the virtual separation of regioisomers of methoxyphenol using SDS implies that differences in diffusion can be ascribed to different relative affinity for the SDS micelles, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values of the compounds in Fig. 10 were evaluated using ChemOffice Chemdraw Ultra 12.22 It could be hypothesised that a greater log
P values of the compounds in Fig. 10 were evaluated using ChemOffice Chemdraw Ultra 12.22 It could be hypothesised that a greater log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P could result in greater affinity for the non-polar component of SDS micelles and result in a reduced observed diffusion coefficient (as originally observed for IPMP). The variation in diffusion coefficient between these three molecules should, therefore correlate approximately to their individual lLog
P could result in greater affinity for the non-polar component of SDS micelles and result in a reduced observed diffusion coefficient (as originally observed for IPMP). The variation in diffusion coefficient between these three molecules should, therefore correlate approximately to their individual lLog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values, however, the diffusion coefficients observed by this group actually show a correlation (R2 = 0.98 of log
P values, however, the diffusion coefficients observed by this group actually show a correlation (R2 = 0.98 of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D to log
D to log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mr) for these three molecule series. Whilst further work will be necessary to determine the factors which contribute the most to the observed change in diffusion coefficient of a lipophilic molecule when in the presence of a surfactant, this preliminary data suggests it may still be possible to predict Mr values based on log
Mr) for these three molecule series. Whilst further work will be necessary to determine the factors which contribute the most to the observed change in diffusion coefficient of a lipophilic molecule when in the presence of a surfactant, this preliminary data suggests it may still be possible to predict Mr values based on log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D data in the presence of surfactants. In mixtures such as these, not only are a small series of known hydrophilic diffusion standards necessary for the establishment of a log
D data in the presence of surfactants. In mixtures such as these, not only are a small series of known hydrophilic diffusion standards necessary for the establishment of a log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D vs. log
D vs. log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mr correlation, but also that a series of lipophilic diffusion standards may be necessary for a reliable extrapolation of Mr values for unknown compounds based on log
Mr correlation, but also that a series of lipophilic diffusion standards may be necessary for a reliable extrapolation of Mr values for unknown compounds based on log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D data. It should be stressed that further studies will need to be conducted to look at the effects of residency time in the micelles and the potential impact of any complexed lipophilic components on shape and thus apparent diffusion.
D data. It should be stressed that further studies will need to be conducted to look at the effects of residency time in the micelles and the potential impact of any complexed lipophilic components on shape and thus apparent diffusion.
4.2. Reliability of qNMR
One of the initial hypothesis was that qNMR was a valid technique for the accurate quantification of organic species in a complex, viscous mixture. Reference to Fig. 7 indicates that pH has a substantial impact on the accuracy of the technique and that proper dispersion of the analyte is essential for correlation. This is reflected in the discrepancy between data shown in Fig. 7 compared to samples containing SDS. Even at pH 9, the IPMP phenoxide is insoluble at high concentrations >0.05% w/w. It should be mentioned that other groups have focused on instrumental uncertainties (type B)30 to account for the observed precision, however for this work, they are largely dwarfed by the inherent error in the weighing balance and the type A uncertainties, so their relevance in this case was questionable. In light of the lipophilicity of the IPMP, SDS was essential for its dispersion and for correlation of the calculated concentration to the amounts added. The accuracy of the qNMR method was 98–99% based on the weighed amounts of IPMP for each % concentration for an SDS/glycerol mixture in PBS, but in the presence of ZnCl2, the accuracy fell to 85% at higher concentrations of IPMP with an increase in error to ±1 mM, suggesting insoluble Zn2+ salt formation over time. The exchange of Zn2+ with Na+ could also affect the size and shape of the SDS micelle, this may result in the promotion of longer rods, rather than spheroids; however, if this is a uniform occurrence throughout the solution it should have equal impact on lipophilic species. Surprisingly the qNMR correlation for IPMP in artificial saliva (Fig. 9) and dentifrice slurry (Fig. 8) showed a good correlation at all concentrations. The accuracy was affected by pH with a lower pH favouring accuracy of 98–99% for IPMP in the dentifrice slurry and a higher pH favouring accuracy of 96–98% in the artificial saliva, SDS, glycerol model. There was no statistically significant difference in the error margins for the qNMR calculation of concentration of IPMP for either of the two systems at low concentration.5. Conclusion
We have demonstrated that DOSY NMR is a useful tool for the characterisation and quantification of natural products as represented by IPMP in systems of variable viscosity and in the presence of surfactants and high salt concentration. Whilst it is important that multiple diffusion standards should be used when attempting to identify unknown components based on predicted Mr from log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D data, the use of qNMR to determine accurately the concentration of dissolved organic shows considerable promise for analysing complex mixtures of natural products. Through the non-destructive identification and quantification of these components, some of the anti-plaque or antimicrobial effects of polyphenolic and terpinoid derivatives could potentially be investigated directly in situ and without the need for separation and the potential loss of key components, which may contribute to this activity. Consistent and accurate quantitative data is possible for these systems and this will facilitate the investigation of the combined impact of individual components with the potential to assist in the development of a combination of natural products with enhanced anti-plaque activity.
D data, the use of qNMR to determine accurately the concentration of dissolved organic shows considerable promise for analysing complex mixtures of natural products. Through the non-destructive identification and quantification of these components, some of the anti-plaque or antimicrobial effects of polyphenolic and terpinoid derivatives could potentially be investigated directly in situ and without the need for separation and the potential loss of key components, which may contribute to this activity. Consistent and accurate quantitative data is possible for these systems and this will facilitate the investigation of the combined impact of individual components with the potential to assist in the development of a combination of natural products with enhanced anti-plaque activity.
Acknowledgements
Thanks go to Dr Jean Marie Peron for excellent NMR technical support and to GSK for funding of ES.References
- C. J. Silwood, E. Lynch, A. W. Claxson and M. C. Grootveld, J. Dent. Res., 2002, 81, 422–427 CrossRef CAS PubMed.
- I. Chinou, Primary and secondary metabolites and their biological activity, in Thin Layer Chromatography in Phytochemistry, ed. M. Waksmundzka-Hajnos, J. Sherma and T. Kowalska, CRC, New York, 2008, pp. 59–76 Search PubMed.
- S. Duarte, S. Gregoire, A. P. Singh, N. Vorsa, K. Schaich, W. H. Bowen and H. Koo, FEMS Microbiol. Lett., 2006, 257, 50–56 CrossRef CAS PubMed.
- H. Koo, S. Duarte, R. M. Murata, K. Scott-Anne, S. Gregoire, G. E. Watson, A. P. Singh and N. Vorsa, Caries Res., 2010, 44, 116–126 CrossRef CAS PubMed.
- S. Shibata, T. Suge, T. Kimura, K. Ishikawa and T. Matsuo, J. Dent., 2012, 25, 31–34 Search PubMed.
- J. G. Jeon, P. L. Rosalen, M. L. Falsetta and H. Koo, Caries Res., 2011, 45, 243–263 CrossRef PubMed.
- L. Cheng, R. A. Exterkate, X. Zhou, J. Li and J. M. Cate, Caries Res., 2011, 45, 87–92 CrossRef CAS PubMed.
- S. Twetman, Caries Res., 2004, 38, 223–229 CrossRef CAS PubMed.
- P. D. Marsh, Br. Dent. J., 2012, 212, 601–606 CrossRef CAS PubMed.
- A. Le Gresley, J. Kenny, C. Cassar, A. Kelly, A. Sinclair and M. Fielder, Food Chem., 2012, 135, 2879–2886 CrossRef CAS PubMed.
- M. Nilsson, P. Aman, H. Harkonen, G. Hallmans, K. E. B. Knudsen, W. Mazur and H. Adlercreutz, J. Sci. Food Agric., 1997, 73, 143–148 CrossRef CAS.
- M. Nilsson, J. Magn. Reson., 2009, 200, 296–302 CrossRef CAS PubMed.
- L. Fielding, Tetrahedron, 2000, 56, 6151–6170 CrossRef CAS.
- J. A. Donarski, D. P. T. Roberts and A. J. Charlton, Anal. Methods, 2010, 2, 1479–1483 RSC.
- R. Consonni and L. R. Cagliani, J. Agri. Food Chem., 2008, 56, 6873–6880 CrossRef CAS PubMed.
- J. S. McKenzie, J. A. Donarski, J. C. Wilson and A. J. Charlton, Prog. Nucl. Magn. Reson. Spectrosc., 2011, 59, 336–359 CrossRef CAS PubMed.
- R. Jaiswal, L. Jayasinghe and N. Kuhnert, J. Mass Spectrom., 2012, 47, 502–515 CrossRef CAS PubMed.
- J. Klimek, E. Hellwig and G. Ahrens, Caries Res., 1982, 16, 156–1616 CrossRef CAS PubMed.
- M. Nilsson, I. F. Duarte, C. U. Almeida, I. Delgadillo and B. J. Goodfellow, J. Agri. Food Chem., 2004, 52, 3736–3743 CrossRef CAS PubMed.
- M. H. Gehlen and F. C. De Schryver, Chem. Rev., 1993, 93, 199–221 CrossRef CAS.
- D. Li, G. Kagan, R. Hopson and P. G. Williard, J. Am. Chem. Soc., 2009, 131, 5627–5634 CrossRef CAS PubMed.
- A. Macchioni, G. Ciancaleoni, C. Zuccaccia and D. Zuccaccia, Chem. Soc. Rev., 2008, 37, 479–489 RSC.
- R. W. Adams, J. A. Aguilar, J. Cassani, G. A. Morris and M. Nilsson, Org. Biomol. Chem., 2011, 9, 7062–7064 CAS.
- C. F. Tormena, R. Evans, S. Haiber, M. Nilsson and G. A. Morris, Magn. Reson. Chem., 2010, 48, 550–553 CrossRef CAS PubMed.
- S. Malz and H. Janke, J. Pharm. Biomed. Anal., 2005, 38, 813–823 CrossRef PubMed.
- N. J. Serkova and M. S. Brown, Bioanalysis, 2012, 4, 321–341 CrossRef CAS PubMed.
- G. F. Pauli, T. Godecke, B. U. Jaki and D. C. Lankin, J. Nat. Prod., 2012, 75, 834–851 CrossRef CAS PubMed.
- M. Weber, C. Hellriegel, A. Ruck, R. Sauermoser and J. Wuthrich, Accredit. Qual. Assur., 2013, 18, 91–98 CrossRef CAS.
- H. Kovacsa, D. Moskaua and M. Spraulb, Prog. Nucl. Magn. Reson. Spectrosc., 2005, 46, 131–155 CrossRef PubMed.
- T. Schoenberger, Anal. Bioanal. Chem., 2012, 403, 247–254 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |