Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Robust ultrasound assisted extraction approach using dilute TMAH solutions for the speciation of mercury in fish and plant materials by cold vapour atomic absorption spectrometry (CVAAS)
M. V. Balarama
Krishna
* and
D.
Karunasagar
National Centre for Compositional Characterization of Materials (NCCCM), Bhabha Atomic Research Centre, Department of Atomic Energy, Hyderabad-500 062, India. E-mail: balaram@cccm.gov.in; Fax: +91 4027125463; Tel: +91 4027121365
First published on 15th January 2015
Abstract
A simple and rapid ultrasound assisted extraction (UAE) protocol with dilute solutions of tetramethylammonium hydroxide (TMAH) for the speciation of mercury in fish and plant tissues was developed as an alternative to conventional methods which require intensive treatments. The main operational parameters, such as extractant concentration (TMAH), sonication time and amount of sample, were optimized using BCR ERM-CE 464 (tuna fish) and mercury loaded coriander powder, an in-house reference material, taken as representatives of fish and plant tissues, respectively. Quantitative extractions of the inorganic mercury (iHg) and methyl mercury (MeHg) species were obtained using 8 mL of 2% TMAH with a sonication time of 5 min for a sample weight of <0.5 g. After sonication, the supernatant obtained upon centrifugation was directly used for the determination of iHg by cold vapour atomic absorption spectrometry (CVAAS). Inorganic mercury was determined using SnCl2 as a reducing agent, while total mercury was determined after the oxidation of methyl mercury (MeHg) with KMnO4 solution. Amount of organic mercury, basically MeHg, was obtained by using the difference. The analytical results were in good agreement with the certified reference values of iHg, MeHg and total mercury at a 95% confidence level. The method was further validated through the analysis of additional certified reference materials: BCR CE-463 (tuna fish), IAEA-350 (fish homogenate), BCR-60 (aquatic plant Lagarosiphon major) and BCR-482 (lichen). The detection limit of the overall procedure was found to be 0.014 μg g−1 for both inorganic and methyl mercury species.
Introduction
Mercury is a global pollutant and highly toxic among heavy metals because of its persistence, long range transport potential and bioaccumulation in the environment. Mercury is introduced in the environment mainly as elemental mercury (Hg0), inorganic mercury (iHg) and organic mercury species as a result of both natural and anthropogenic activities from where it re-enters the human food chain.1–7 More than 2500 tons of mercury is emitted annually from global anthropogenic sources, which are significantly contributing to elevated levels of mercury. It has been known that organomercury compounds, particularly methyl mercury (MeHg), are 50–100 times more toxic than inorganic mercury species.8 These two are the common and predominant forms of mercury generally found in biological and environmental samples such as fish tissues and plant matrices.9–13 Because of the accumulative properties and adverse toxic effects of mercury species even at ultra-trace levels, its accurate determination in fish and plant samples is very important for environmental protection and food safety.As a consequence, considerable efforts and progress have been made in the development of sensitive and accurate sample preparation methods for the determination of total mercury and its speciation analysis in environmental and biological samples.14–26 The most frequently used approaches for the extraction of mercury species from fish and plant samples are based on microwave27–30 or ultrasound31,32 assisted alkaline or acid leaching and solid phase extraction.33,34 Despite excellent sensitivity and selectivity, most of the abovementioned approaches suffer from major limitations that include laboriousness of the procedures, use of high amount of acids along with complexing agents, lack of acceptable efficiency and long time consumption.
TMAH and formic acid reagents have been extensively used as the most appropriate tissue solubilizers for various biological samples prior to the analysis of various elements including mercury and its speciation.35–40 Among these two solubilizers, the alkaline solubilization with TMAH offers a simple and rapid approach for the preparation of a homogenized sample solution, which is a distinct advantage over conventional slurry preparation methods. Therefore, several methods for the determination of iHg and MeHg species using TMAH have been developed and reported in the literature.35–38,41–43 However, sample solutions produced after solubilisation with TMAH are cloudy and emit an unpleasant odour as well that requires adequate ventilation. The use of dilute TMAH solutions can minimize the odour, but quantitative extraction of the species of interest may be affected.
Nowadays, there have been significant developments in green analytical methodologies aimed to reduce the amount of toxic chemical reagents as well as simplify and accelerate experimental procedures.44–46 In this context, the ultrasound assisted extraction (UAE) approach can be an excellent alternative to minimize the abovementioned limitations of conventional extraction procedures.47,48 Being a clean technology, ultrasound energy has already been well exploited for a number of analytical applications such as speeding up solid–liquid extraction of the elements/species of interest for the determination of total-element contents and speciation analysis, remediation, organic synthesis and a number of other analytical and industrial applications.49–53 Based on these facts, ultrasound assisted extraction protocol was utilized in the present work for the speciation of mercury in fish and plant materials using dilute TMAH solutions.
The most commonly and widely used techniques employed for the determination of mercury species in a great variety of matrices, including fish and plant tissues with and without applying chromatographic separation, are cold vapour atomic absorption spectrometry (CVAAS)54 and atomic fluorescence spectrometry (CVAFS).55 In the present study, CVAAS was selected for Hg determination because of its high sensitivity, absence of spectral interferences, relatively low operational costs and simplicity as well as rapidity.
The main objective of the work is to develop a simple, efficient and green analytical methodology for the determination of t-Hg, iHg and indirectly MeHg without the use of a chromatographic separation after treatment with dilute TMAH solutions with the aid of ultrasound probe energy, which is suitable for both fish and plant tissues. Mercury loaded coriander powder (representative of samples of plant origin) and BCR CRM 464 (tuna fish; representative of fish) were used for optimization experiments. After extraction using optimized conditions, the concentration of iHg and tHg were determined using CVAAS after employing KMnO4 treatment for the oxidation of organic mercury species to inorganic mercury. A closed microwave digestion procedure based on the use of dilute nitric acid solutions and H2O2 was utilized for the dissolution of the test samples for the subsequent determination of total mercury by CVAAS.
Experimental
Instrumentation
Reagents and materials
All the chemicals used in this work were at least of AR grade. High-purity water with a resistivity of >18 MΩ cm, used for the preparation of standards and samples and for the cleaning of vessels, was produced using a Milli-Q high purity water system, located in class 100 area of the ultra-trace analysis laboratory of this centre. Dilute solutions of TMAH, prepared from stock solution (25% in methanol, Aldrich, USA), was used as the extractant. Tin(II) chloride (SnCl2) (5%, w/v) used as the reducing agent was prepared by dissolving an appropriate amount of SnCl2·2H2O (Merck, India) in HCl and diluting with water. Sodium borohydride (NaBH4) (Merck, Darmstadt, Germany) (1%, w/v) was prepared fresh daily by dissolving an appropriate amount of solid in 0.3% (w/v) NaOH solution. A carrier solution of 10% HCl was used along with SnCl2 or NaBH4 for the reduction of mercury. Inorganic mercury standard solution (1000 mg L−1) in 5% HNO3 (SD Fine-Chem Ltd, Mumbai, India) traceable to NIST 3133 was used as a stock standard. A methyl mercury (CH3Hg+) stock standard solution (100 mg L−1, Hg as MeHg) was prepared from methyl mercury iodide (Aldrich) by dissolving an appropriate amount of the solid in methanol and making up to the required volume with high purity water. All the stock standard solutions were stored in a refrigerator at 4 °C and protected from light. Working standard solutions were prepared just before use by the appropriate dilution of the stock standard solutions.The following certified reference materials (CRMs) were analysed to evaluate the developed method; Lichen-482 from Community Bureau of Reference (BCR), BCR-60 (Lagarosiphon major, aquatic plant), European reference Materials (ERM) CE-463 and 464 (tuna fish) and fish homogenate IAEA-350. All the solid reference materials were used as received, without further grinding and sieving.
A large quantity of coriander plants was obtained from the local market and thoroughly washed with water to remove all the adhering soil particles. The whole plant (roots, stem and leaves) was cut into small pieces and dried at 50 °C in a conventional heating oven, ground in a planetary ball mill (Fritz, Germany) and sieved to get a particle size of ≤100 μm. After this step, about 10 g of the powdered coriander was placed in a glass beaker containing 200 mL of high purity water spiked with 100 μg of iHg and MeHg individually (designated as Cori-iHg and Cori-MeHg, respectively) such that the amount of mercury species in the coriander compounded to about 10 μg g−1 Hg in the solution.
The mixture was continuously stirred for about 1 h for quantitative sorption and to facilitate uniform loading of spiked mercury. After shaking, the mixture was separated by centrifugation (8000 rpm for 5 min) and the supernatant was drained. Then, the sorbent was initially allowed to dry at room temperature and then dried in a conventional heating oven at ∼40 °C to remove the residual moisture. Then, the dried sample was finely ground and sieved to obtain 200–400 mesh size particles. In another set of experiments, both iHg and MeHg (10 μg each) were loaded on coriander powder (weight of coriander 10 g) using a procedure similar to the one described above such that the total amount of mercury in coriander was about 20 μg g−1 (Cori-iHg–MeHg). In all the cases, the supernatant was analysed for the determination of residual mercury by CVAAS, and the results indicated the absence of mercury.
For total Hg determination, an accurately weighed aliquot (∼200 mg) of the target materials was placed in the PTFE microwave digestion vessel to which 1 mL of concentrated HNO3 and 1 mL of H2O2 followed by 2 mL of high purity water were added. After closing, the vessels were clamped within a support module and placed inside a microwave-assisted digestion system. A microwave program consisting of the following steps was used: (i) temperature was ramped to 100 ± 2 °C in 5 min (pre-digestion step); (ii) temperature was ramped to 200 ± 5 °C in 10 min and held there for 10 min and, (iii) zero power for 20 min (cooling step). After cooling, the resultant clear sample digests were quantitatively transferred from the PTFE vessel to another pre-cleaned tube and diluted to the desirable volume with water depending on the concentration of mercury. After suitable dilution, all the sample solutions were analysed by CVAAS after VG of mercury using SnCl2 and/or NaBH4 for the determination of the total mercury present in each CRM. The corresponding process blank solutions were also subjected to the same procedure in the absence of the sample.
A known amount of iHg standard/sample solution was added into a reaction vessel (of CVAAS system) containing ∼5 mL of 10% HCl carrier solution. The reaction mixture was vigorously stirred for the desired length of time (1–3 min) in a closed environment before passing Hg0 vapors to the quartz cell of the AAS system for quantification. One part of the split samples was analysed for the determination of iHg using SnCl2 as the selective reducing agent. To determine total Hg, it was necessary to add an appropriate amount of KMnO4 solution to the other part of the split sample for oxidation treatment in the presence of 5% HNO3 to convert MeHg to iHg, which was followed by its determination by CVAAS using SnCl2 or NaBH4 as the reducing agent. The concentration of methyl mercury was calculated as the difference between the total and iHg values.
Corresponding process blanks (with and without oxidative treatment) were also prepared in the same way without taking any sample material. Three different aliquots of each sample were used for the extraction process. All the analytical measurements were run in triplicate for each sample solution. With each series of extractions, a blank was also prepared and measured in parallel to determine the cross-contamination of mercury. Quantifications of the mercury species in the test samples are based on a 5-point calibration graph obtained with the standards of mercury in the concentration range of 0 (analytical blank)-100 ng mL−1 prepared using process blank solutions containing 2% TMAH and TMAH-extracted blank coriander sample solutions. These calibration plots were compared with those of pure aqueous standards of mercury to test the matrix effects, if any. The standard addition method was also applied to determine other possible interferences, if any.
To test the volatility of the mercury species under ultrasound-assisted extraction conditions, a set of experiments was carried out, in which standards containing known amounts of mercury species (iHg and MeHg) prepared in 8 mL of the optimized extractant solution (2% TMAH) were subjected to the proposed ultrasound-assisted extraction procedure as in the case of the samples. The resultant solutions (after suitable dilution) were analysed for the determination of the species of mercury by CVAAS. Calibration plots were also obtained with these processed standard solutions and compared with the plots obtained for pure aqueous mercury standards.
After applying the ultrasound-assisted extraction process, the extraction efficiency at each step was tested by calculating the percentage recovery of the test mercury species in the samples using the following equation:
Results and discussion
The main concerns in the quantitative extraction of the mercury species from solid matrices (in this case fish and plant tissues) should be the efficiency, volatility, inter-species conversion, contamination and amount of reagents required. Extraction methods based on the use of ultrasound energy usually do not require intensive conditions such as high temperatures, pressures or concentrated acids. Based on this fact, the present work was initiated using dilute solutions of TMAH with the aid of ultrasound energy for the speciation of mercury. As mentioned in earlier sections, inorganic and methyl mercury (MeHg) species are the two common and predominant forms generally found in various biological and environmental samples. Therefore, the present study was focussed on the determination of only inorganic and methyl mercury species.Initially, a series of experiments were carried out to optimize these variables for quantitative recovery of both iHg and MeHg. Mercury loaded coriander powder (representative of samples of plant origin) and BCR CRM 464 (tuna fish; representative of fish tissue) were used for the optimization experiments. In the case of the fish representative sample, the concentration of iHg was very low (represented only 2.3% of the total Hg concentration); thus, the level of iHg was increased using standard addition to evaluate the stability of both iHg and MeHg species during the USE process. Accordingly, ∼0.2 g of ERM-CE464 was spiked with 100 μL of iHg standard (from 10 μL mL−1 stock standard), to which extraction solvent TMAH was added. After each extraction step, the percentage recovery of both iHg and MeHg was determined during the method development.
Total mercury determination
Different volumes of HNO3 and H2O2, irradiation times and microwave power settings of CEM microwave system were tested to ensure the total recovery of Hg. In each case, ∼200 mg of the solid sample was taken and digested using the microwave program described in the earlier section. The addition of a mixture of 1.5 mL HNO3, 1 mL of H2O2 and 2.5 mL of water greatly improved the efficiency of digestion, providing a clear solution and quantitative recovery of mercury from the CRMs, selected in this work. The reduction of mercury was carried out using NaBH4 with a concentration of 2% w/v for the subsequent determination of total mercury by CVAAS. The results obtained with the digestion performed with the proposed procedure were found to be in good agreement with the certified values (recoveries higher than 98%). The use of diluted HNO3 in the presence of H2O2 was proven to be a feasible and recommendable sample digestion procedure complying with the green chemistry recommendations.Speciation analysis of mercury
The ultrasound-assisted extraction of mercury species may not be equally effective for all the solid samples; thus, maximizing the extraction yield requires the process variables to be optimized for each specific matrix (in this case, plant and fish matrices). The extraction efficiency of ultrasound energy is essentially governed by various parameters including extractant concentration (TMAH), sonication time and amount of sample. Therefore, these variables were individually optimized to achieve quantitative recovery of both the mercury species, while the others were kept constant.Optimization of concentration of TMAH
As mentioned in the earlier sections, TMAH is strongly alkaline, soluble in aqueous media, stabilizes volatile elements and does not require heating or only requires gentle heating, and it is thus promising for the speciation analysis of mercury. In the present work, dilute solutions of TMAH were used to test its efficacy as an extractant to achieve the quantitative extraction of the mercury species from plant and fish tissues with the aid of ultrasound energy. Based on the results obtained from various preliminary experiments, different concentrations of TMAH in the range of 0.5–3% were chosen for two representative materials, keeping the other parameters (sonication time: 5 min, volume of extractant: 8 mL and amount of sample: ∼200 mg) constant. An extractant volume of 8 mL was chosen in all the optimization experiments so that the required number of replicates could be performed without the exhaustion of the sample solution.As a compromise between sensitivity and reagent consumption, 5% w/v SnCl2 in 10% v/v HCl solution was chosen as the reducing agent for the determination of iHg, while 2% w/v NaBH4 and 5% v/v HNO3 was chosen as the optimum condition for tHg determination in the final TMAH-sample extracts after oxidation treatment with KMnO4.
Fig. 1a and b shows the effect of the concentration of TMAH on the extraction efficiency of the iHg and MeHg species from BCR CRM 464 and mercury loaded coriander representative materials. As shown in these figures, extraction efficiency (i.e., recovery of Hg species from the solid matrix) with water (in the absence of TMAH) was very low (<10%), while the efficiency of TMAH for the extraction of both iHg and MeHg increased with the concentration of TMAH up to 2% and reached a plateau in the concentration range of 2–5%, which is the highest studied concentration. As seen from Fig. 1a and b, the optimum concentration of TMAH was found to be about 1.5% for the quantitative extraction (>95%) of the two selected mercury species from BCR-464, while 2% of TMAH was required for the mercury loaded coriander material (which had a plant origin). In general, fish tissues are soft as compared to plant tissues, and thus fish tissue requires a lower concentration of TMAH for the complete extraction of the species of interest.
Both iHg and MeHg species show similar extraction behaviour with quantitative recoveries between 95% and 102% when dilute solutions of TMAH were used as the extractant. After sonication, the colour of the final extractant solution resembled the original colour of the powdered sample. The effect of ultrasound energy on the stability of the Hg species was also studied using the two representative materials by analyzing TMAH-extracted solutions at different time intervals. These studies clearly indicate that after carrying out UAE with 2% TMAH, the two tested mercury species remained stable even after standing for a week in the laboratory at room temperature. The TMAH concentration of 2% v/v was adopted for further extraction experiments to make it suitable to both fish and plant tissues.
Optimization of sonication time
The sonication time of the sample is an important parameter because the dose of ultrasound sonication received by the matrix and extractant mixture determines the extent of cavitation phenomena followed by the efficiency of extraction. The sonication time of 5 min or less is usually reported when ultrasonic probes are used for solid liquid extraction. Fixing ultrasound amplitude (40%), extractant concentration (2% v/v TMAH), extractant volume (8 mL) and sample weight (∼200 mg), we investigated the influence of sonication time on the extraction of the Hg species in the range of 1–6 min. In both the fish and the coriander representative samples, the extraction efficiency of the two Hg species increased from 45% to ∼98% as the sonication time increased from 1 to 4 min and remained almost constant in time interval of 5–7 min. The results obtained from these studies indicated that the sonication time of 4 min was found to be sufficient for the quantitative extraction of mercury species from both the representative materials, which is advantageous to obtain a high sample throughput. A sonication time of 5 min was thus selected as the optimum for further optimization studies because the species recovery was highly reproducible.Evaluation of KMnO4 concentration and reaction time for MeHg oxidation
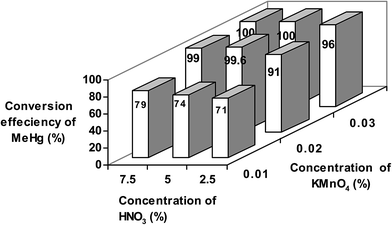
First, the concentrations of KMnO4 and HNO3 were optimized for the quantitative conversion of MeHg to Hg2+ followed by CVAAS determination. This oxidation treatment was performed before adding a reducing agent for VG of mercury. As mentioned above, iHg was determined using SnCl2 as the selective reducing agent, whereas tHg was determined after the oxidation of organic mercury to iHg through reaction with KMnO4 followed by reduction to elemental mercury. A variety of oxidizing agents, viz., H2O2, KMnO4, K2Cr2O7 and K2S2O8, in combination with strong acids (such as HCl and HNO3), UV and microwave irradiation have been extensively used for the oxidation of organic mercury to iHg followed by the determination of tHg. In the present work, KMnO4 was selected to decompose the organomercury species (predominantly MeHg in this case) due to its ease of preparation, stability and low mercury blank. KMnO4 also promotes the efficient stabilization of mercury in solution until analysis.41Because the extraction of the mercury species was carried out using a 2% TMAH solution, it is necessary to add HNO3 along with KMnO4 to acidify the sample digest for the rapid oxidation of the organomercury species. Methyl mercury loaded coriander sample (Cori-MeHg) and tuna fish (BCR-CE 464) were taken as representatives for optimizing the concentration of HNO3 and KMnO4 required for the quantitative conversion of CH3Hg+ to Hg2+. After a general speciation procedure, a sample volume of 0.5 mL was taken for the optimization studies. To optimize the composition of HNO3 and KMnO4, a factorial (two factors, three levels) experimental design approach was applied and the conversion efficiency of MeHg at each level of treatment was estimated. Based on the results obtained from various preliminary experiments, a mixture of 4.5 mL of 0.02% w/v KMnO4 and 5% v/v HNO3 (added to a reaction vessel of CVAAS containing 0.5 mL of the TMAH-extracted sample) was selected as the base level for the two representative materials (the upper and lower levels were obtained using a difference of ±0.01% for KMnO4 and ±2.5% for HNO3). The mixture was stirred for about 1 min, and then the reducing agent was added for the determination of mercury by CVAAS. At each optimization step, the corresponding solutions were employed as blanks.
From Fig. 2, it can be seen that the conversion efficiency of MeHg varied significantly with different concentrations of KMnO4 and HNO3 added to the TMAH-extracted coriander sample solution. From these studies, it was observed that the best efficiency of conversion was obtained with a mixture of 4.5 mL of 0.02% w/v KMnO4 and 5% v/v HNO3 for 0.5 mL of the sample solution. This is believed to be a result of the efficient conversion of MeHg to Hg2+ in the standard and samples as well as due to the stabilization of mercury in the standard/sample solution in its oxidized form. Similar results were obtained for the fish representative sample, and thus the data obtained for fish sample are not shown here.
In the case of the MeHg standard, the addition of a mixture of 4.5 mL of 0.01% w/v KMnO4 and 5% v/v HNO3 allowed quantitative conversion to iHg, whereas the conversion efficiency was only 70–80% for TMAH-extracted sample solutions. Thus, it was inferred that more oxidizing agent is required for test samples in comparison with the MeHg standard solution because of the presence of other sample components, which competed with the MeHg species during the oxidation process. This may be mainly due to the consumption of a major part of KMnO4 by the sample matrix, thus reducing the availability of the oxidizing agent for the oxidative conversion of CH3Hg+ to Hg2+. Based on these results, a mixture of 4.5 mL of 0.02% w/v KMnO4 and 5% v/v HNO3 was added to the reaction vessel (of CVAAS) containing 0.5 mL of the sample solution prior to the reduction to elemental mercury. However, for treating a higher volume of TMAH-extracted sample solutions (>0.5 mL) (depending on the concentration of MeHg), an increased amount of KMnO4 solution is required for the quantitative conversion.
After optimizing the concentration of the oxidizing agent KMnO4, it was necessary to optimize the reaction time (stirring time) required for a complete oxidation of the CH3Hg+ to Hg2+ in the tested samples. Based on a series of experiments, a reaction time of 1 min was chosen as the optimum because the recovery of mercury was quantitative and the mercury signal was highly reproducible. No significant improvement in sensitivity could be obtained with longer reaction periods (1–3 min). Thus, a reaction time (i.e., stirring time) of 1 min was used in all the subsequent experiments.
Tao et al.36 used L-cysteine and KMnO4 for the determination of iHg and tHg, respectively. They added L-cysteine to sample solutions to liberate iHg from protein-bound mercury or other molecules in the TMAH-extracted solutions. In this work, however, the addition of L-cysteine did not enhance the iHg level, indicating that the reducing agent (SnCl2 or NaBH4) alone was found to be sufficient (without the need of L-cysteine) for the quantitative recovery of iHg in the sample solutions after UAE using dilute TMAH (∼2%) solutions.
Figures of merit
The whole analytical procedure proposed for the speciation of mercury in plant and fish tissues is schematically presented in Fig. 3. Calibration curves were obtained across the concentration range of 0 (analytical blank)-100 ng mL−1 for iHg and MeHg species prepared in different solvent media (aqueous, 2% TMAH and TMAH-extracted solutions of blank coriander powder). Analytical response characteristics of iHg and MeHg species spiked in different solvent media are presented in Table 1. In all the cases, the correlation coefficients were >0.995. The slopes of the calibration curves corresponding to Milli-Q water, 2% TMAH solutions and TMAH-extracted sample solutions spiked with iHg and MeHg did not differ significantly, showing no matrix effect in the TMAH medium, and thus demonstrating the efficacy of the developed UAE procedure using dilute solutions of TMAH. This allows the use of the aqueous standard calibration curve for quantification purposes. As both the external and standard addition approaches provided comparable results, all mercury measurements were subsequently carried out using only the external calibration method.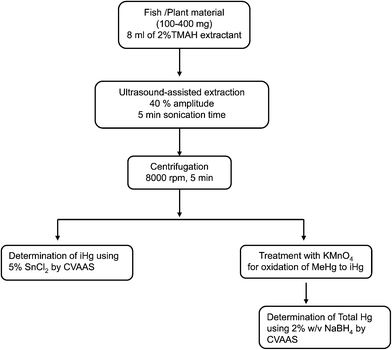 | ||
| Fig. 3 Schematic flow diagram of the proposed ultrasound-assisted extraction method for the analysis of total mercury and its species from various fish and plant matrices. | ||
| Medium | Response function | |
|---|---|---|
| Hg2+ spiked | CH3Hg+ spikedb | |
| a Calibration points – 10, 25, 50, 75, 100 ng mL−1. b Determined after KMnO4 treatment. | ||
| Aqueous medium | y = 0.048x − 0.079, R2 = 0.996 | y = 0.047x − 0.067, R2 = 0.998 |
| 2% TMAH medium | y = 0.050x + 0.037, R2 = 0.995 | y = 0.049x + 0.029, R2 = 0.996 |
| TMAH-extracted coriander sample (blank) solution | y = 0.047x + 0.029, R2 = 0.997 | y = 0.046x + 0.033, R2 = 0.995 |
The analytical results of the mercury loaded coriander sample and various CRMs together with the certified/reference values are presented in Table 2 and 3, respectively. The determined values for total iHg obtained by both UAE and MAD digestion methods agree with the certified values (at 95% confidence level). The organic mercury concentration, calculated as the difference between the total and iHg values, also agrees with the certified MeHg concentration. This demonstrates that most of the organic mercury obtained by the arithmetic difference is mainly MeHg. The detection limit of the method, determined as the concentration corresponding to three times the standard deviation of the blank, was 0.014 μg g−1 based on 0.4 g of the sample and 8 mL of the extractant solution. The precision, evaluated as the relative standard deviation (RSD%), was better than 10% in most of the cases.
| Sample type | Loaded values (mg kg−1) | Values obtained with the developed UAE method (mg kg−1) | MW digestion (mg kg−1) | |||
|---|---|---|---|---|---|---|
| Hg2+ | CH3Hg+ | Hg2+ | #CH3Hg+ | Total-Hg | Total mercury | |
| Coriander powder (blank) | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| Inorganic mercury loaded coriander powder | 10 | — | 10.8 ± 0.5 | <LOD | 10.3 ± 0.2 | 10.5 ± 0.4 |
| Methyl mercury loaded coriander powder | — | 10 | <LOD | 10.1 ± 0.5 | 10.6 ± 0.3 | 9.7 ± 0.5 |
| Mixture of inorganic and methyl mercury loaded coriander powder | 10 | 10 | 10.1 ± 0.4 | 9.7 ± 0.3 | 19.8 ± 0.4 | 20.3 ± 0.8 |
| Type of reference material | Certified values (mg kg−1) | Obtained in this work (mg kg−1) | MW digestion (mg kg−1) | |||
|---|---|---|---|---|---|---|
| Total-Hg | CH3Hg+ | Total-Hg | CH3Hg+a | Hg2+ | Total-Hg | |
| a Values calculated as difference between total mercury and inorganic mercury. b LOD = Limit of detection. | ||||||
| Lagarosiphon major BCR-60 aquatic plant | 0.34 ± 0.04 | <LOD | 0.33 ± 0.03 | <LOD | 0.35 ± 0.02 | 0.35 ± 0.03 |
| Lichen BCR-482 | 0.48 ± 0.02 | <LOD | 0.50 ± 0.04 | <LOD | 0.49 ± 0.03 | 0.47 ± 0.05 |
| Fish homogenate IAEA-350 | 4.68 ± 0.28 | 3.65 ± 0.35 | 4.65 ± 0.21 | 3.74 ± 0.19 | 0.91 ± 0.05 | 4.65 ± 0.22 |
| Tuna fish ERM-CE 463 | 2.85 ± 0.16 | 3.04 ± 0.16 | 2.92 ± 0.13 | 2.88 ± 0.12 | 0.04 ± 0.01 | 2.93 ± 0.12 |
| Tuna fish ERM-CE 464 | 5.24 ± 0.10 | 5.50 ± 0.17 | 5.36 ± 0.12 | 5.24 ± 0.11 | 0.12 ± 0.02 | 5.28 ± 0.13 |
The proposed analytical procedure markedly reduces the concentration of TMAH required for extraction by more than 10 times as compared to the reported solubilisation methods and the time needed for sample preparation (total 10 min including centrifugation time). In addition, keeping the number of analytical steps to a minimum considerably reduces the sources of analytical errors.
Conclusions
An effective analytical method based on the use of a dilute TMAH (2%) solution as the extractant with the aid of ultrasound energy for the speciation analysis of mercury by CVAAS in plant and fish tissues was developed. The developed extraction procedure and Hg-species determination was validated by the analysis of various certified reference materials. After ultrasound-assisted extraction, the TMAH-extracted sample solutions were directly analysed for iHg by CVAAS, while tHg was determined after oxidation with a solution of KMnO4. This method also provides very important information on the toxic organomercury content, mainly MeHg (determined as the difference between tHg and iHg) in fish and plant tissues without handling a highly toxic methyl mercury standard. If a sample contains other organic species, such as phenyl mercury and dimethyl mercury, then the present method shall be suitable only for the identification of the inorganic and organic forms of mercury. The developed method can not only significantly reduce the sample preparation time, but also provide quantitative recoveries (in the range of 95–102%) and preserve the integrity of the species. In addition, extra reagents (such as L-cysteine) and concentrated reagents (TMAH) are not required for the determination of iHg and total mercury. In the proposed UAE approach, the speciation analysis of mercury was achieved without using any chromatographic technique, requiring only an ultrasound probe and CVAAS instruments. The main features of the present UAE method are as follows: no matrix separation, reduction in time and solvent consumption, easy implementation, efficacy, reproducibility and safety of the procedure.Acknowledgements
The authors are thankful to Dr Sunil Jai Kumar, Head, NCCCM for his constant support and encouragement.References
- W. L. Clevenger, B. W. Smith and J. D. Winefordner, Crit. Rev. Anal. Chem., 1997, 27, 1–26 CrossRef CAS.
- D. W. Boening, Ecological Effects, transport and fate of mercury; a general review, Chemosphere, 2000, 40, 1335–1351 CrossRef CAS.
- J. Brent, Clin. Toxicol., 2001, 39/7, 707–710 CrossRef PubMed.
- P. Li, X. B. Feng, G. L. Qiu, L. H. Shang and Z. G. Li, J. Hazard. Mater., 2009, 168, 591–601 CrossRef CAS PubMed.
- N. E. Selin, J. Environ. Monit., 2011, 13, 2389–2399 RSC.
- M. Leermarkers, W. Baeyens, P. Quevauviller and M. Horvat, Trends Anal. Chem., 2005, 24/5, 383–393 CrossRef PubMed.
- K. A. Francesconi, Analyst, 2007, 132, 17–20 RSC.
- J. C. A. de Wuilloud, R. G. Wuilloud, R. A. Olsinaa and L. D. Martinez, J. Anal. At. Spectrom., 2002, 17, 389–394 RSC.
- C. F. Harrington, TrAC, Trends Anal. Chem., 2000, 19/2+3, 167–179 CrossRef.
- C. M. Tseng, A. De Diego, F. M. Martin, D. Amouroux and O. F. X. Donard, J. Anal. At. Spectrom., 1997, 12, 743–750 RSC.
- M. Ruiz-de-Cenzano, A. Rochina-Marco, M. L. Cervera and M. De la Guardia, Ecotoxicol. Environ. Saf., 2014, 107, 90–96 CrossRef CAS PubMed.
- M. J. Sierra, R. Millan and E. Esteban, Food Chem. Toxicol., 2009, 47, 2716–2767 CrossRef PubMed.
- J. Hellings, S. B. Adeloju and T. V. Verheyen, Microchem. J., 2013, 111, 62–66 CrossRef CAS PubMed.
- R. Clough, C. F. Harrington, S. J. Hill, Y. Madrid and J. F. Tyson, J. Anal. At. Spectrom., 2014, 29, 1158–1196 RSC.
- J. J. B. Nevado, R. C. R. Martin-Doimeadios, E. M. Krupp, F. J. G. Bernardo, N. R. Farinas, M. J. Moreno, D. Wallace and M. J. P. Ropero, J. Chromatogr. A, 2011, 1218, 4545–4551 CrossRef PubMed.
- C. Ibanez-Palomino, J. F. Lopez-Sanchez and A. Sahuquillo, Anal. Chim. Acta, 2012, 720, 9–15 CrossRef CAS PubMed.
- Y. YongGuang, L. JingFu and J. GuiBin, Chin. Sci. Bull., 2013, 58/2, 150–161 Search PubMed.
- J. L. Capelo-Martinez, P. Ximenez-Embun, Y. Madrid and C. Camara, TrAC, Trends Anal. Chem., 2004, 23/4, 331–340 CrossRef.
- M. V. Balarama Krishna, A. C. Sahayam and D. Karunasagar, Anal. Methods, 2012, 4, 210–216 RSC.
- M. V. Balarama Krishna, K. Chandrasekaran and D. Karunasagar, Talanta, 2010, 81, 462–472 CrossRef CAS PubMed.
- A. L. C. Ortiz, Y. M. Albarran and C. C. Rica, J. Anal. At. Spectrom., 2002, 17, 1595–1601 RSC.
- Z. Yun, B. He, Z. Wang, T. Wang and G. Jiang, Talanta, 2013, 106, 60–65 CrossRef CAS PubMed.
- M. A. Vieira, A. S. Ribeiro, A. J. Curtius and R. E. Sturgeon, Anal. Bioanal. Chem., 2007, 388, 837–847 CrossRef CAS PubMed.
- S. Rio Segade and J. F. Tyson, Spectrochim. Acta, Part B, 2003, 58, 797–807 CrossRef.
- L. H. Reyes, G. M. Rahman, T. Fahrenholz and H. M. Skip Kingston, Anal. Bioanal. Chem., 2008, 390, 2123–2132 CrossRef CAS PubMed.
- L. Carrasco and E. Vassileva, Talanta, 2014, 122, 106–114 CrossRef CAS PubMed.
- C. S. Eskilsson and E. Bjorklund, J. Chromatogr. A, 2000, 902, 227–250 CrossRef CAS.
- L. H. Reyes, J. L. G. Mar, A. Hernandez-Ramirez, J. M. Peralta-Hernandez, J. M. A. Barbosa and H. M. Skip Kingston, Microchim. Acta, 2011, 172, 3–14 CrossRef CAS.
- L. H. Reyes, G. M. M. Rahman and H. M. Skip Kingston, Anal. Chim. Acta, 2009, 631, 121–128 CrossRef CAS PubMed.
- L.-F. Chang, S.-J. Jiang and A. C. Sahayam, J. Chromatogr. A, 2007, 1176, 143–148 CrossRef CAS PubMed.
- S. Rio-segade and C. Bedicho, J. Anal. At. Spectrom., 1999, 14, 263–268 RSC.
- M. V. Balarama Krishna, M. Ranjit, D. Karunasagar and J. Arunachalam, Talanta, 2005, 67, 70–80 CrossRef PubMed.
- S. Diez and J. M. Bayona, Talanta, 2008, 77, 21–27 CrossRef CAS PubMed.
- A. R. Turker, D. Cabuk and O. Yalcinkaya, Anal. Lett., 2013, 46/7, 1155–1170 CrossRef.
- G. Tao, S. N. Willie and R. E. Sturgeon, Analyst, 1998, 123, 1215–1218 RSC.
- G. Tao, S. N. Willie and R. E. Sturgeon, J. Anal. At. Spectrom., 1999, 14, 1929–1931 RSC.
- D. P. Torres, V. L. A. Frescura and A. J. Curtius, Microchem. J., 2009, 93, 206–210 CrossRef CAS PubMed.
- C. J. Park and H. Do, J. Anal. At. Spectrom., 2008, 23, 997–1002 RSC.
- I. Serafimovski, I. Karadjova, T. Stafilov and J. Cvetkovic, Microchem. J., 2008, 89, 42–47 CrossRef CAS PubMed.
- H. Matusiewicz and E. Stanisz, Cent. Eur. J. Chem., 2010, 8(3), 594–660 CrossRef CAS PubMed.
- D. P. Torres, D. L. G. Borges, V. L. A. Frescura and A. J. Curtius, J. Anal. At. Spectrom., 2009, 24, 1118–1122 RSC.
- M. Kan, S. N. Willie, C. Scriver and R. E. Surgeon, Talanta, 2006, 68, 1259–1263 CrossRef CAS PubMed.
- C. Scriver, M. Kan, S. Willie, C. Soo and H. Birnboim, Anal. Bioanal. Chem., 2005, 381, 1460–1466 CrossRef CAS PubMed.
- Y. Wu, Y.-I. Lee, L. Wu and X. Hou, Microchem. J., 2012, 103, 105–109 CrossRef CAS PubMed.
- M. De la Guardia, TrAC, Trends Anal. Chem., 2010, 29/7, 577 CrossRef PubMed.
- D. L. Rocha, A. D. Batista, F. R. P. Rocha, G. L. Donati and J. A. Nobrega, TrAC, Trends Anal. Chem., 2013, 45, 79–92 CrossRef CAS PubMed.
- C. Bendicho, I. De La Calle, F. Pena, M. Costas, N. Cabaleiro and I. Lavilla, TrAC, Trends Anal. Chem., 2012, 31, 50–60 CrossRef CAS PubMed.
- Y. Pico, TrAC, Trends Anal. Chem., 2013, 43, 84–89 CrossRef CAS PubMed.
- H. M. Santos and J. L. Capelo, Talanta, 2007, 73, 795–802 CrossRef CAS PubMed.
- C. Bendicho, I. Lavilla, F. Pena-Pereira and V. Romero, J. Anal. At. Spectrom., 2012, 27, 1831–1857 RSC.
- S. Gil, I. Lavilla and C. Bendicho, Anal. Chem., 2006, 78, 6260–6264 CrossRef CAS PubMed.
- A. S. Ribeiro, M. A. Vieira, S. Willie and R. E. Sturgeon, Anal. Bioanal. Chem., 2007, 388, 849–857 CrossRef CAS PubMed.
- M. V. Balarama Krishna and J. Arunachalam, Anal. Chim. Acta, 2004, 522, 179–187 CrossRef PubMed.
- C. E. Oda and J. D. Ingle Jr, Anal. Chem., 1981, 53, 2305–2309 CrossRef CAS.
- D. Sanchez-Rodas, W. T. Corns, B. Chen and P. B. Stockwell, J. Anal. At. Spectrom., 2010, 25, 933–946 RSC.
- D. Karunasagar, M. V. Balarama Krishna, S. V. Rao and J. Arunachalam, J. Hazard. Mater., 2005, B118, 133–139 CrossRef PubMed.
- C. A. Bizzi, E. M. M. Flores, R. S. Picoloto, J. S. Barin and J. A. Nobrega, Anal. Methods, 2010, 2, 734 RSC.
- C. A. Bizzi, E. M. M. Flores, J. A. Nobrega, J. S. S. Oliveira, L. Schmidt and S. R. Mortari, J. Anal. At. Spectrom., 2014, 29, 332–338 RSC.
| This journal is © The Royal Society of Chemistry 2015 |