Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Regal electrochemistry: British 5 pence coins provide useful metallic macroelectrode substrates†
Fang
Tan
,
Jamie P.
Smith
,
Dimitrios K.
Kampouris
,
Joanna
Kamieniak
and
Craig E.
Banks
*
Faculty of Science and Engineering, School of Science and the Environment, Division of Chemistry and Environmental Science, Manchester Metropolitan University, Chester Street, Manchester M1 5GD, UK. E-mail: c.banks@mmu.ac.uk; Web: http://www.craigbanksresearch.com Fax: +44 (0)161-247-6831; Tel: +44 (0)161-247-1196
First published on 18th August 2015
Abstract
The utilisation of British Currency (GBP) as an electrode substrate is demonstrated for the first time. Termed Regal electrochemistry, a 5 pence (5p) coin (GBP) is electrically wired using a bespoke electrochemical cell and is electrochemically characterised using the outer-sphere redox probe hexaammineruthenium(III) chloride. The electroanalytical utility of the 5p coin electrode is demonstrated towards the novel, proof-of-concept sensing of lead(II) ions using square-wave voltammetry in model buffer solutions over the linear range 5–2000 nM exhibiting a limit of detection (3σ) of 1.97 nM. Interestingly, the actual cost of the electrode is 2.5 pence (GBP) since both sides of the coins can be utilised and provide a cheap yet reproducible and disposable metallic electrode substrate that is electrochemically useful.
Introduction
Electroanalysts are always searching for new materials that are both economical and electroanalytically useful. To this end, a wide array of different materials are explored in the hope of finding the next low cost, reliable sensor that, if applicable, is useable in-the-field (disposable) and exhibits useful electroanalytical performances towards the target analyte.One analyte that demands attention is the sensing of lead(II) ions which continues to be one of the most problematic toxic heavy metals that has caused environmental contamination and health problems around the world.1–3 It acts as a cumulative toxicant that affects different body systems including the neurological, haematological, gastrointestinal, cardiovascular and renal systems. Children are particularly vulnerable to the neurotoxic effects of lead, even at even relatively low levels of exposure, potentially causing serious and irreversible neurological damage.1,3 As a result of its negative health affects, lead(II) ions are regulated by the World Health Organisation (WHO) to a maximum of 10 ppb (48.26 nM) within drinking water4 mirrored by the United States Environmental Protection Agency (EPA) which states no higher than 15 ppb (72.39 nM) in drinking water.5 Clearly, there is a need for the on-site detection of lead(II) ions using portable, inexpensive and sensitive analytical methods – electrochemical techniques provide a potential solution. In the literature there are various attempts at the electrochemical quantification of lead(II) ions2,6–14 such as DNA electrodes,6 carbon nanotubes,8 carbon ionic liquid electrodes,9 screen-printed sensors12 and reduced graphene oxide modified screen-printed sensors.14
In this communication, it is demonstrated for the first time, that British coinage, namely a 5 pence (GBP) coin, can be used as a novel electrical substrate. For this new branch of electrochemistry the phrase “Regal electrochemistry” has been coined. Through the use of information readily available from the Royal mint website,15 the composition of the current coins in circulation is known and presented in Table 1.15 The British 5 pence (GBP) coin offers the potential to be a low cost electrochemical sensing platform that is reproducible and particularly useful as the coin is two sided, placing its value at just 2.5 pence per sensor. This is primarily useful when the electroanalytical protocol is intended to be implemented into third world countries where the realisation of low cost, reliable sensors is imperative to applications such as heavy metals testing nevertheless, there is no reason to suggest that ‘Regal’ electrochemical sensors cannot be ‘coined’ for other analytical applications.
| Coin | Composition |
|---|---|
| 1p | Bronze (97% copper, 2.5% zinc, 0.5% tin) – until September 1992 |
| Copper-plated steel – since September 1992 | |
| 2p | Bronze (97% copper, 2.5% zinc, 0.5% tin) – until September 1992 |
| Copper-plated steel – since September 1992 | |
| 5p | Pre-2012 copper–nickel (cupro–nickel alloy) 75% copper, 25% nickel |
| Post-2012 Nickel-plated steel – since January 2012 | |
| 20p | Pre-2012 copper–nickel (cupro–nickel alloy) 75% copper, 25% nickel |
| Nickel-plated steel – since January 2012 | |
| 50p | Cupro–nickel (84% copper, 16% nickel) |
| £1 | Cupro–nickel (75% copper, 25% nickel) |
| £2 | Outer: nickel–brass (76% copper, 4% nickel, 20% zinc) |
| Inner: cupro–nickel (75% copper, 25% nickel) | |
Results and discussion
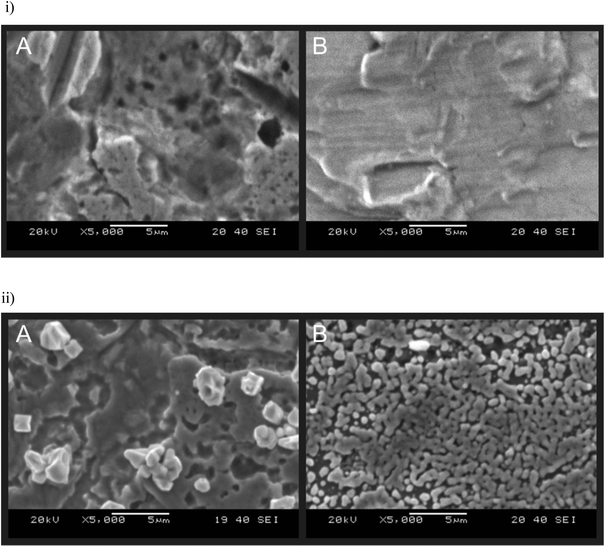
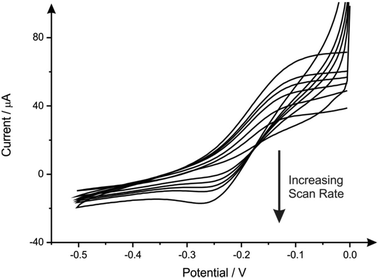
British coinage was obtained and treated as described in the experimental section (see ESI†) with which to test the theory that coins can be potentially utilised as novel electrode substrates; to the best of our knowledge, there are no reports using coins as electrochemical substrates. To test the coins electrochemical reactivity towards the detection of lead(II) ions, two coins, held within a bespoke Polytetrafluoroethylene (PTFE) ‘housing unit’ (see ESI Fig. 1†), were subject to ‘electrolysis’ to allow the ions to deposit on the surface. To this end, the two coins were independently held at a suitable negative potential (−1.2 V) in an aqueous pH 4 acetate buffer solution containing 1000 nM Pb(II) (lead(II) nitrite) to allow the deposition of lead, in the form of lead metal, onto the coins surface to occur. Fig. 1 shows scanning electron microscope (SEM) images of the two coins before and after deposition. Note: Scanning Electron Microscope – Energy-Dispersive X-ray microanalysis (SEM-EDX) was employed as confirmatory analysis to demonstrate the coins contained no impurities and to show if lead was present on the surface following electrodeposition. It was also utilised to confirm the sheet nickel metal used (see later) was pure nickel. Discernible from Fig. 1 is that the deposition of metallic lead shows a much higher affinity of adsorption on the 5p coin minted post-2012 (Fig. 1(ii)B) opposed to the surface of coins minted prior to 2012 (Fig. 1(ii)A). As previously mentioned, in information readily available from the United Kingdom's Royal Mint, the composition of a 5p coin since January 2012 has been nickel plated steel and prior to this date it was a copper–nickel alloy (cupro–nickel); see Table 1. This suggests, as it is present in both, lead(II) binds to the nickel present in the coins/upon the coins surface. A secondary result of this is; as the lead is actively deposited on nickel this technique could be used for mapping nickel-based alloys in light of its selectivity towards nickel over other metals.In light of its higher nickel content (and therefore higher affinity for lead deposition) the electrochemical performance of the post-2012 5 pence (5p) coin electrode (held in the custom-made PTFE ‘housing unit’; see ESI Fig. 1†) was explored first using the outer-sphere redox probe 1 mM hexaammineruthenium(III) chloride/0.1 M KCl over the scan rate range 5–200 mV s−1. Fig. 2 shows the obtained voltammetric response and clearly observable is the process of electron transfer wherein a reduction process occurs at approximately −0.25 V and an oxidation process at −0.1 V: this is indicative of the potential use of a 5p coin as an electrochemical substrate. A pre-2012 5p coin was also tested in 1 mM hexaammineruthenium(III) chloride/0.1 M KCL and is found to yield a poor electrochemical response (ESI Fig. 2†). A large cathodic wave is observed at ∼−0.2 V using the pre-2012 5p coin which is similar to that reported for copper reduction as previously reported in the literature.16 Coins minted pre-2012 have a large composition of copper (75%) that is, mostly likely, in the form of an oxide such as Cu2O or CuO. In light of the composition, the following processes likely occur:
| Cu(aq)2+ + 2e−(m) ⇌ Cu(s) |
| Cu(aq)+ + e−(s) ⇌ Cu(s) |
From this it can be readily deduced that the electrochemical reduction of copper will dominate the electrochemical response as opposed to the desirable reduction of lead(II) on pre-2012 5p coins (as is evident from Fig. 1) and as such, all experiments performed were with the use of a coin minted post-2012.
A control experiment was performed using a possible alternative to the potential coin electrodes. Visible in ESI Fig. 3,† confirmation of nickel's electroactivity towards lead(II) by using pure nickel sheet metal (contained within the PTFE ‘housing unit’) towards a 48.3 nM solution of lead(II). Whilst there is a measurable response, it comes with a much greater financial outlay (25 mm × 25 mm sheets can have costs in excess of £100 (ref. 17)) and it is not as prevalent as the readily available 5 pence coin. Regal electrochemistry is also beneficial when compared to using nickel plated metal which have been used as electrode substrates18 as the plating can be a long process and not everybody has access to the specialist equipment required to perform such techniques however, everybody in the United Kingdom has access to low denominations of its currency.
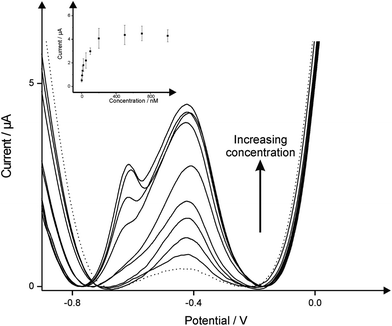
Having determined that 5p coins minted post-2012 offered the best sensor apropos of lead ion determination its electroanalytical performance was explored. A series of additions were made over the analytical range 5–1000 nM into a pH 4 acetate buffer solution; the square wave voltammograms obtained utilizing the 5p-coin through the additions of lead are illustrated in Fig. 3. Note that at high concentrations the electrode surface is likely saturated accounting for the plateau observed in the insert of Fig. 3 and the additional pre-peak. Analysis of the calibration curves (Fig. 3 inset), constructed using the oxidation peak at approximately −0.40 V reveal a limit of detection (3σ) equal to 1.97 nM which is within the WHO guidelines of 10 ppb (48.26 nM) in drinking water.1,4 In light of this, the presented avant-garde, proof-of-concept ‘Regal electrochemistry’ square-wave voltammetric approach is a much cheaper, facile method for the detection of lead as opposed to other key advancements within the area.19–21 Note that faces on the coins have reliefs. In our experiments both sides of the coin are utilised; ESI Fig. 4† shows the two sides of the coinage used in this work. This potentially adds to the surface “roughness” however, this is negligible since on the timescale of the experiment the diffusion layer is larger than the “roughness”. Note that both sides of coins have been used and used to provide error bars presented in the inset of Fig. 3.
In summary we have demonstrated that coinage can be successful utilised as electrode sensor. Further work will see other coins explored electrochemically for their response to an array of analytes to advance the area of Regal electrochemistry.
Conclusions
Proof-of-concept that British coinage can be successfully utilised as a useful electrode material has been shown with detection of lead in the nM range with model solutions. This has been compared to high quality (pure) nickel sheet metal and due to its significant cost has no advantages over the use of coinage as an electrode material – the use of a 5 pence incurs the cost of just 2.5 pence since both sides can be readily utilised. Note that the use of coinage as an electrode material is an attractive proposition since everyone will have a coin in his or her pocket, which can be readily utilised at low cost with minimal pretreatment and in light of its low cost – can be used as a one shot sensor. This is particularly useful in both developing countries and emergency cases such as those postured in militaristic applications. From inspection of Table 1, one can readily see that other coins have favorable compositions that could also be potentially used as electrode substrates. The concept of Regal electrochemistry can be expanded to other coinage towards a range of target (electroactive) analytes; such work is currently underway.References
- World Health Organization, International Programme on Chemical Safety: Lead, http://www.who.int/ipcs/assessment/public_health/lead/en/, accessed April 2015.
- W. Yantasee, Y. Lin, K. Hongsirikarn, G. E. Fryxell, R. Addleman and C. Timchalk, Environ. Health Perspect., 2007, 115, 1683–1690 CrossRef CAS PubMed.
- R. A. Goyer, Toxicology of metals - biochemical aspects, Springer-Verlag, New York, 1995 Search PubMed.
- WHO, Guidance for Drinking Water Quality, Recommendations, Geneva, 2nd edn, 1993, vol. 1 Search PubMed.
- United States Environmental Protection Agency, Safe Water Drinking Act: Levels of Copper and Lead, http://water.epa.gov/lawsregs/rulesregs/sdwa/lcr/index.cfm, accessed June 2015.
- Y. Xiao, A. A. Rowe and K. W. Plaxco, J. Am. Chem. Soc., 2007, 129, 262–263 CrossRef CAS PubMed.
- G. Mai, L. Xia and W. Wang, Russ. J. Electrochem., 2013, 49, 447–452 CrossRef CAS.
- Y. Zhu, G.-m. Zeng, Y. Zhang, L. Tang, J. Chen, M. Cheng, L.-h. Zhang, L. He, Y. Guo, X.-x. He, M.-y. Lai and Y.-b. He, Analyst, 2014, 139, 5014–5020 RSC.
- H.-J. Liu, L.-N. Qu, S. Hu, T.-R. Zhan, C.-Z. Zhao and W. Sun, J. Chin. Chem. Soc., 2010, 57, 1367–1373 CrossRef CAS PubMed.
- A. Wanekaya and O. A. Sadik, J. Electroanal. Chem., 2002, 537, 135–143 CrossRef CAS.
- I. V. Anambiga, V. Suganthan, N. A. N. Raj and A. S. Kumar, Electrochemical sensor for the detection of lead ions, 2013 Search PubMed.
- A. Mandil, L. Idrissi and A. Amine, Microchim. Acta, 2010, 170, 299–305 CrossRef CAS.
- A. P. Ruas de Souza, C. W. Foster, A. V. Kolliopoulos, M. Bertotti and C. E. Banks, Analyst, 2015, 140, 4130–4136 RSC.
- J.-M. Jian, Y.-Y. Liu, Y.-L. Zhang, X.-S. Guo and Q. Cai, Sensors, 2013, 13, 13063–13075 CrossRef CAS PubMed.
- The Royal Mint, Coin Design and Specifications: Five Pence Coin, http://www.royalmint.com/discover/uk-coins/coin-design-and-specifications/five-pence-coin, accessed April 2015.
- D. Stankovic, G. Roglic, J. Mutic, I. Andjelkovic, M. Markovic and D. Manojlovic, Int. J. Electrochem. Sci., 2011, 6, 5617 CAS.
- Goodfellow Supplier of Materials for Research and Development, http://www.goodfellow.com/E/Nickel-Foil.html, accessed July 2015.
- P. Y. Chen, H. H. Yangi, J. M. Zen and Y. Shih, J. AOAC Int., 2011, 94, 1585–1591 CrossRef CAS.
- B. Chen, Z. Wang, D. Hu, Q. Ma, L. Huang, C. Xv, Z. Guo and X. Jiang, Sens. Actuators, B, 2014, 200, 310–316 CrossRef CAS PubMed.
- G. Lisak, J. Bobacka and A. Lewenstam, J. Solid State Electrochem., 2012, 16, 2983–2991 CrossRef CAS.
- E. Kirowa-Eisner, M. Brand and D. Tzur, Anal. Chim. Acta, 1999, 385, 325–335 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5an01218j |
| This journal is © The Royal Society of Chemistry 2015 |