Open Access Article
Open Access ArticleElectrogenerated chemiluminescence of tris(2,2′ bipyridine)ruthenium(II) using common biological buffers as co-reactant, pH buffer and supporting electrolyte†
Noah
Kebede
a,
Paul S.
Francis
*b,
Gregory J.
Barbante
b and
Conor F.
Hogan
*a
aDepartment of Chemistry and Physics, La Trobe Institute for Molecular Science, La Trobe University, Melbourne, Victoria 3086, Australia. E-mail: C.Hogan@latrobe.edu.au
bCentre for Chemistry and Biotechnology, School of Life and Environmental Sciences, Faculty of Science, Engineering and Built Environment, Deakin University, Waurn Ponds, Victoria 3216, Australia. E-mail: paul.francis@deakin.edu.au
First published on 28th August 2015
Abstract
A series of aliphatic tertiary amines (HEPES, POPSO, EPPS and BIS-TRIS) commonly used to buffer the pH in biological experiments, were examined as alternative, non-toxic co-reactants for the electrogenerated chemiluminescence (ECL) of tris(2,2′-bipyridine)ruthenium(II) ([Ru(bpy)3]2+). These were found to be very attractive as “multi-tasking” reagents, serving not only as co-reactants, but also fulfiling the roles of pH buffer and supporting electrolyte within an aqueous environment; thus significantly simplifying the overall ECL analysis. Sub-nanomolar detection limits were obtained for [Ru(bpy)3]2+ in the presence of BIS-TRIS, making this species an valuable option for co-reactant ECL-based bioanalytical applications.
Introduction
Electrogenerated chemiluminescence (ECL) is the emission of light from the excited products of a chemical reaction, where at least one reactant is generated electrochemically.1–3 The first detailed studies of ECL were described by Hercules4 and Bard et al.5 in the mid-1960s; however, reports of light emission during electrolysis date back to the 1920s.6 In 1972, Tokel and Bard7 described the ECL of [Ru(bpy)3]2+via annihilation between oxidised and reduced forms of the complex in acetonitrile. Bard's group also demonstrated the first co-reactant ECL (using oxalate),8 before Leland and Powell9 introduced tri-n-propylamine (TPrA) in 1990. Blackburn et al.10 subsequently adopted this system for ECL detection in immunoassays using [Ru(bpy)3]2+-based labels.TPrA remains by far the most widely used co-reactant for ECL,3,11–19 and the [Ru(bpy)3]2+/TPrA system is currently employed in the vast majority of commercially available ECL instrumentation and methods.1,20
TPrA is an effective co-reactant for [Ru(bpy)3]2+ ECL, but there are several well-known problems associated with its use. Most importantly, TPrA is highly toxic (LD50 oral: 98 mg Kg−1, LC50 inhalation: 1500 mg m−3) and quite volatile. It is destructive to the mucous membrane and upper respiratory tract system of the human body and can be fatal if inhaled. TPrA is not readily soluble in water, but relatively high concentrations (∼100 mM) are required to attain the highest sensitivity.11–13 Furthermore, the intensity of [Ru(bpy)3]2+ ECL with TPrA as the co-reactant strongly depends on the working electrode material (for example, intensities generated using Pt electrodes are only 10% of the those at Au electrodes).21
Not surprisingly, there has been considerable interest in the development of alternatives to TPrA for co-reactant ECL.21,22 Most notably, Liu et al.21 introduced 2-(dibutylamino)ethanol (DBAE) as a safer co-reactant for [Ru(bpy)3]2+ ECL. Under certain circumstances, such as at relatively low co-reactant concentrations when using glassy carbon working electrodes, DBAE gave greater ECL intensities than TPrA, but under other conditions, the novel co-reactant is not as effective. Han et al.22 subsequently examined a series of tertiary amines and ethanolamines, and reported that N-butyldiethanolamine performed better than DBAE at lower co-reactant concentrations, but unfortunately, it is more toxic than DBAE.
Several commonly used laboratory buffers (Fig. 1) possess similar chemical structures to previously investigated tertiary amine and ethanolamine co-reactants.16,17 Moreover, Leland and Powell9 reported that when triethanolamine and 1,4-piperazinediethanesulfonic acid (PIPES) were used as co-reactants with [Ru(bpy)3]2+, the ECL intensity was 53% and 31% of that using TPrA, respectively. Later, a study exploring the co-reactant ability of a number of biological buffers in the presence of an electrolyte23 showed that such species could elicit ECL from [Ru(bpy)3]2+.
In this work we have carried out a detailed study of the potential of several common laboratory buffers as alternative co-reactants for [Ru(bpy)3]2+ ECL. These buffers are a sub-set of those referred to as ‘biological’ buffers, or ‘Good’ buffers, after the pioneering work on these substituted glycine and N-substituted taurine molecules by Good and co-workers.24–26 Importantly, some of the criteria used by Good et al. in the development of these buffers25 are also highly desirable properties for ECL co-reactants, particularly in bioanalytical applications: (i) they should be freely dissolvable in water and should not be able to permeate biological membranes; (ii) they should alter the ionic strength of the system as little as possible; (iii) their pKa should be influenced as little as possible by their concentration, temperature and the ion composition of the medium; (iv) they should not be subject to enzymatic or non-enzymatic changes; (v) they should not be able to absorb light at wavelengths longer than 230 nm; (vi) they should be easily manufactured and purified; and finally (vii) they should be non-toxic and cost effective.
In this study, four ‘Good’ buffers that possess aliphatic tertiary amine/ethanolamine groups (Fig. 1) were examined as alternative, non-toxic co-reactants for [Ru(bpy)3]2+ ECL, with the interesting prospect of also simultaneously serving as the pH buffer and electrolyte within the aqueous environment. A buffer for ECL-based bioanalysis, which performs multiple functions (co-reactant/buffer/electrolyte) would significantly simplify the analytical procedure, reducing the variability associated with adding numerous components to a sample. Moreover, this simplicity would reduce the probability of a reagent interfering with interactions between biomolecules in immunoassays or DNA probe analysis. Finally, replacing the electrolyte with the co-reactant offers the possibility of migrationally enhanced ECL signals.
Materials and methods
Chemicals and materials
Unless otherwise stated, deionised water (Sartorius Stedim biotech arium® pro VF Ultrapure Water System, 18.2 MΩ cm, Germany) and analytical grade reagents were used. The Good buffers shown in Fig. 1: BIS-TRIS hydrochloride, POPSO sesquisodium salt, EPPS, and HEPES sodium salt were obtained from Sigma-Aldrich (NSW, Australia). Tris(2,2′-bipyridine)ruthenium(II) chloride hexahydrate ([Ru(bpy)3]Cl2·6H2O, 99%) was purchased from Strem Chemicals (MA, USA). In the examination of optimum pH, the buffers were each prepared at 0.1 M, with 1 μM [Ru(bpy)3]2+. Useful pH range: BIS-TRIS HCl: pH 5.8–7.2; POPSO sesquisodium salt: pH 7.2–8.5; HEPES sodium salt: pH 6.8–8.2; EPPS: pH 7.3–8.7. The pH was adjusted using 0.1 M HCl or 0.1 M NaOH.Instrumentation
The pH of the working solutions were measured using a MEP Instruments Metrohm 827 pH Lab pH meter and a MEP Instruments Metrohm 6.0228.010 pH electrode. A CH instruments (TX, USA) electrochemical workstation was used to perform cyclic voltammetry experiments (660E) with chi660e software. A custom-built light-tight faraday cage encased the electrochemical cell, which consisted of a cylindrical glass cell with a quarts window base and a Teflon cover with spill tray. A conventional three-electrode configuration was used, comprising a glassy carbon (3 mm diameter) working electrode shrouded in Teflon (CH Instruments, Austin, TX, USA), a 1 cm2 gold wire auxiliary electrode and a Ag/AgCl (3 M KCl) reference electrode (CH Instruments, Austin, TX, USA). The working electrode was polished with alumina slurry on a felt pad, rinsed with water and acetone and dried while under a steady stream of nitrogen. The surface of the working electrode was then positioned at a reproducible distance (∼2 mm) from the bottom of the cell for detection. The ECL intensity was measured with a photomultiplier tube (model 9828SB; Electron Tubes, Ruislip, UK), biased at 500 V using a PM28B power supply (Electron Tubes). The PMT signal was amplified using a TA-GI-74 Ames Photonics amplifier (model D7280) and acquired using the auxiliary channel of the potentiostat. Cyclic voltammetry (CV) was performed over the range from 0 V to 1.5 V at a scan rate of 0.05 V s−1 while the ECL signal was simultaneously recorded.Results and discussion
‘Good’ buffer as a co-reactant
We initially examined the co-reactant ECL intensity of 1 μM [Ru(bpy)3]2+ with each of the four amines shown in Fig. 1 and TPrA at a concentration of 10 mM in aqueous solution, using a phosphate buffer to control the pH and serve as the electrolyte (Fig. 2). The ECL intensity using the biological buffers as co-reactants was between 13% and 48% than using TPrA. | ||
| Fig. 2 Relative ECL intensities for the four amines shown in Fig. 1, with [Ru(bpy)3]2+ compared to that of TPrA, under conventional co-reactant ECL conditions, in 0.1 M phosphate buffer (pH 7). The co-reactant concentration was 10 mM in each case and the concentration of the ruthenium complex was 1 μM. | ||
‘Good’ buffer as a co reactant, buffer and electrolyte
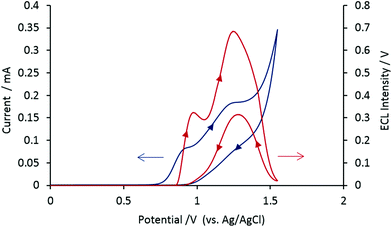
Having shown that these amines can act as efficient co-reactants, we examined the possibility that they could also simultaneously serve as the buffer and the electrolyte in solution (which is not feasible with TPrA). The voltammetric and corresponding co-reactant ECL signals for aqueous solutions containing only [Ru(bpy)3]2+ and each of these tertiary-amine ‘Good’ buffers (i.e. no additional buffer or electrolyte) is shown in Fig. S1, ESI† (EPPS, HEPES and POPSO) and Fig. 3 (BIS-TRIS HCl).Effect of pH
The ECL intensity of [Ru(bpy)3]2+ (1 μM) with each biological buffer in aqueous solution was examined across the useful pH range of the buffer (Fig. S2, ESI†). Under these conditions, the optimum pH for co-reactant ECL was 8.0 for HEPES sodium salt, 8.4 for POPSO sesquisodium salt, 8.3 for EPPS and 5.8 for BIS-TRIS HCl. The peak ECL intensities for [Ru(bpy)3]2+ with biological buffer as co-reactant, buffer and electrolyte increased in the order: POPSO sesquisodium salt (0.39 V) < EPPS (0.43 V) < BIS-TRIS HCl (0.69 V) < HEPES sodium salt (0.77 V). These ECL signals were lower than that obtained with TPrA as co-reactant and a phosphate buffer/electrolyte (9.98 V), but as shown below can still provide detection limits sufficiently low for many biological assays. Moreover, they offer a useful alternative in terms of the safety and simplicity of sample preparation.The mechanism of co-reactant ECL of [Ru(bpy)3]2+ and tertiary-amine biological buffers
Considering the similarity between the chemical structure of the biological buffers under investigation (Fig. 1) and the aliphatic tertiary amine/ethanolamine co-reactants such as TPrA and DBAE, a mechanism for the ECL of [Ru(bpy)3]2+ with these four biological buffers can be confidently drawn from previous investigations.27,28 Although the reaction may proceed simultaneously via several pathways,27 under conditions involving low concentrations of the luminophore and relatively high concentrations of the co-reactant in aqueous solution, the dominant pathway can be illustrated as follows:| [Ru(bpy)3]2+−e− → [Ru(bpy)3]3+ | (1) |
| B−e− → B˙+ | (2) |
| B˙+ → B˙ + H+ | (3) |
| [Ru(bpy)3]3+ + B˙ → [Ru(bpy)3]2+* + other products | (4) |
| [Ru(bpy)3]2+* → [Ru(bpy)3]2+ + hν | (5) |
Both [Ru(bpy)3]2+ and the tertiary-amine biological buffer (B) are oxidised at the electrode surface forming [Ru(bpy)3]3+ and a radical cation of the buffer. This radical cation becomes deprotonated and the neutral radical reduces [Ru(bpy)3]3+, enabling excited state formation ([Ru(bpy)3]2+*) which emits a photon to return to the ground state.
With this in mind, the lower co-reactant ECL intensities from the four biological buffers (Fig. 2) compared to that of TPrA can in part be rationalised. Three of the biological buffers (EPPS, POPSO and HEPES) are diamines. The CV of each system shows relatively large cathodic current, indicative of significant side reactions at the electrode surface. In the case of diamines, it is possible that their oxidation generates intermediates that contain both an oxidative amine cation radical (B˙+) and a reductive amine free radical (B˙), leading to intramolecular reactions that consume the key intermediates required to generate the excited state (i.e.eqn (4)).22 Han et al. previously reported that monoamines DBAE and N-butyldiethanolamine gave greater co-reactant ECL intensities than the closely related diamine molecules such as N,N,N′,N′-tetrakis-(2-hydroxyethyl)ethylenediamine.22 The difference in ECL intensity using BIS-TRIS HCl as a co-reactant compared to TPrA (or DBAE) is more difficult to explain. Subtle changes in co-reactant structure can have a dramatic effect on ECL intensity.29 Under specific experimental conditions, certain aliphatic tertiary amines containing one or two β-hydroxyl substituents (DBAE21 or N-butyldiethanolamine22) have been found to be more effective co-reactants than TPrA, but triethanolamine (containing three β-hydroxyl substituents) gave lower ECL intensities than DBAE.22,29
Determination of [Ru(bpy)3]2+ using BIS-TRIS simultaneously as co reactant, buffer and electrolyte
A calibration of ECL intensity versus [Ru(bpy)3]2+ concentration (Fig. 4), with 0.1 M BIS-TRIS HCl serving as co-reactant, buffer and electrolyte, revealed a limit of detection of 0.2 nM (S/N = 2). Obtaining sub-nanomolar detection limits for [Ru(bpy)3]2+ employing a biological buffer as co-reactant, buffer and electrolyte opens up new possibilities for immunoassays, DNA probe assays and cellular imaging applications, in which the use of the toxic and volatile TPrA can be avoided.Conclusions
The results presented show that the aliphatic tertiary amine/ethanolamine ‘Good’ buffers can be employed as multi-tasking reagents in ECL-based assays; serving as co-reactant, buffer and electrolyte in ECL systems (using [Ru(bpy)3]2+ as the luminophore) over a wide pH range. Although the biological buffers give lower ECL intensities compared with TPrA, this is compensated for by ease of sample preparation due to their higher aqueous solubility and the simplicity advantage of requiring fewer reagents. Moreover, their lower volatility and considerably lower toxicity allow for a safer and more environmentally friendly analysis and waste disposal. Therefore, although these buffers are not likely to replace traditional ECL co-reactants such as TPrA, they do provide a useful alternative for certain applications where exceedingly low detection limits are not required.Notes and references
- L. Hu and G. Xu, Chem. Soc. Rev., 2010, 39, 3275–3304 RSC.
- R. J. Forster, P. Bertoncello and T. E. Keyes, Annu. Rev. Anal. Chem., 2009, 2, 359–385 CrossRef CAS PubMed.
- W. Miao, Chem. Rev., 2008, 108, 2506–2553 CrossRef CAS PubMed.
- D. M. Hercules, Science, 1964, 145, 808–809 CAS.
- K. S. V. Santhanam and A. J. Bard, J. Am. Chem. Soc., 1965, 87, 139–140 CrossRef CAS.
- R. T. Dufford, D. Nightingale and L. W. Gaddum, J. Am. Chem. Soc., 1927, 49, 1858–1864 CrossRef CAS.
- N. E. Tokel and A. J. Bard, J. Am. Chem. Soc., 1972, 94, 2862–2863 CrossRef CAS.
- I. Rubinstein and A. J. Bard, J. Am. Chem. Soc., 1981, 103, 512–516 CrossRef CAS.
- J. K. Leland and M. J. Powell, J. Electrochem. Soc., 1990, 137, 3127–3131 CrossRef CAS PubMed.
- G. F. Blackburn, H. P. Shah, J. H. Kenten, J. Leland, R. A. Kamin, J. Link, J. Peterman, M. J. Powell, A. Shah, D. B. TaHey, S. K. Tyagi, E. Wilkins, T.-G. Wu and R. J. Massey, Clin. Chem., 1991, 37, 1534–1539 CAS.
- A. W. Knight and G. M. Greenway, Analyst, 1996, 121, 101R RSC.
- W.-Y. Lee, Mikrochim. Acta, 1997, 127, 19–39 CrossRef CAS.
- M. M. Richter, Chem. Rev., 2004, 104, 3003–3036 CrossRef CAS PubMed.
- A. W. Knight, TrAC, Trends Anal. Chem., 1999, 18, 47–62 CrossRef CAS.
- E. H. Doeven, E. M. Zammit, G. J. Barbante, C. F. Hogan, N. W. Barnett and P. S. Francis, Angew. Chem., Int. Ed., 2012, 51, 4354–4357 CrossRef CAS PubMed.
- G. J. Barbante, P. S. Francis, C. F. Hogan, P. R. Kheradmand, D. J. D. Wilson and P. J. Barnard, Inorg. Chem., 2013, 52, 7448–7459 CrossRef CAS PubMed.
- G. J. Barbante, C. F. Hogan, A. Mechler and A. B. Hughes, J. Mater. Chem., 2010, 20, 891 RSC.
- T. Joshi, G. J. Barbante, P. S. Francis, C. F. Hogan, A. M. Bond, G. Gasser and L. Spiccia, Inorg. Chem., 2012, 51, 3302–3315 CrossRef CAS PubMed.
- T. Joshi, G. J. Barbante, P. S. Francis, C. F. Hogan, A. M. Bond and L. Spiccia, Inorg. Chem., 2011, 50, 12172–12183 CrossRef CAS PubMed.
- X. Zhou, D. Zhu, Y. Liao, W. Liu, H. Liu, Z. Ma and D. Xing, Nat. Protoc., 2014, 9, 1146–1159 CrossRef CAS PubMed.
- X. Liu, L. Shi, W. Niu, H. Li and G. Xu, Angew. Chem., Int. Ed., 2007, 46, 421–424 CrossRef CAS PubMed.
- S. Han, W. Niu, H. Li, L. Hu, Y. Yuan and G. Xu, Talanta, 2010, 81, 44–47 CrossRef CAS PubMed.
- J.-P. Choi, Dissertation, University of Texas, 2003.
- N. E. Good, G. D. Winget, W. Winter, T. N. Connolly, S. Izawa and R. M. M. Singh, Biochemistry, 1966, 5, 467–477 CrossRef CAS.
- N. E. Good and S. Izawa, Methods Enzymol., 1972, 24, 53–68 CAS.
- W. J. Ferguson, K. I. Braunschweiger, W. R. Braunschweiger, J. R. Smith, J. J. McCormick, C. C. Wasmann, N. P. Jarvis, D. H. Bell and N. E. Good, Anal. Biochem., 1980, 104, 300–310 CrossRef CAS.
- W. Miao, J.-P. Choi and A. J. Bard, J. Am. Chem. Soc., 2002, 124, 14478–14485 CrossRef CAS PubMed.
- C. M. Hindson, G. R. Hanson, P. S. Francis, J. L. Adcock and N. W. Barnett, Chem. – Eur. J., 2011, 17, 8018–8022 CrossRef CAS PubMed.
- G. J. Barbante, N. Kebede, C. M. Hindson, E. H. Doeven, E. M. Zammit, G. R. Hanson, C. F. Hogan and P. S. Francis, Chem. – Eur. J., 2014, 20, 14026–14031 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5an01216c |
| This journal is © The Royal Society of Chemistry 2015 |