Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A novel reflectance-based aptasensor using gold nanoparticles for the detection of oxytetracycline†
Ho Bin
Seo
a,
Young Seop
Kwon
a,
Ji-eun
Lee
a,
David
Cullen
b,
Hongseok (Moses)
Noh
*c and
Man Bock
Gu
*a
aDepartment of Biotechnology, College of Life Sciences and Biotechnology, Korea University, Anam-dong, Seongbuk-Gu, Seoul 136-713, Republic of Korea. E-mail: mbgu@korea.ac.kr
bCranfield Biotechnology Centre, Institute of BioScience and Technology, Cranfield University at Silsoe, Silsoe, Bedfordshire, UK
cDepartment of Mechanical Engineering and Mechanics, Drexel University, 3141 Chestnut St., Philadelphia, PA 19104, USA. E-mail: mosesnoh@coe.drexel.edu
First published on 14th August 2015
Abstract
We present a novel reflectance-based colorimetric aptasensor using gold nanoparticles for the detection of oxytetracycline for the first time. It was found that the reflectance-based measurement at two wavelengths (650 and 520 nm) can generate more stable and sensitive signals than absorbance-based sensors to determine the aggregation of AuNPs, even at high AuNP concentrations. One of the most common antibacterial agents, oxytetracycline (OTC), was detected at concentrations as low as 1 nM in both buffer solution and tap water, which was 25-fold more sensitive, compared to the previous absorbance-based colorimetric aptasensors. This reflectance-based colorimetric aptasensor using gold nanoparticles is considered to be a better platform for portable sensing of small molecules using aptamers.
Introduction
Antibiotics contained in foods and drinking water have potentially serious effects on human health. Overuse of antibiotics can contribute to the development of antibiotic resistance such as evolving drug-resistant bacteria. Thus, there is a growing demand for on-site diagnosis of antibiotic residues in foods and drinking water. Oxytetracycline (OTC) is one of the antibiotics of the tetracycline group, and used to treat infections caused by Mycoplasma organisms and Chlamydia, preventing infections and diseases of livestock and poultry via intramuscular or oral administration.1 It can damage calcium rich organs such as bones and teeth, causing gastrointestinal and photosensitive allergic reactions.Diverse analytical methods have been investigated to realize rapid on-site detection of antibiotic residues such as OTC from small amounts of food samples. Y-channel microfluidic devices using latex microspheres, electrochemical aptasensors, and aptasensors using gold nanoparticles have been reported for the application.2–5 Some of the most promising sensors among them are colorimetric aptasensors using gold nanoparticles (AuNPs) because of their simple operation and easy detection with the naked eye.4 The previously reported colorimetric aptasensors use either AuNPs modified with partly complementary oligonucleotides for hybridizing with aptamers6,7 or unmodified AuNPs on which single stranded DNA (ssDNA) aptamers can be physically adsorbed.8 The aptasensors using unmodified AuNPs do not require any pre-treatment, and thus they are considered as a better approach for on-site sensing applications. When the target is added to the aptasensor, the target molecules will bind to the aptamers, resulting in the detachment of the adsorbed aptamers from AuNPs. The AuNPs will then aggregate, leading to the color change from red to purple (the absorbance peak shifts from 520 to 650 nm) because the localized surface plasmon resonance (LSPR) is changed.9 This absorbance peak shift can be detected by measuring the peak shift on reflectance signals.10
However, all of the previously reported colorimetric aptasensors using AuNPs are based on the indirect absorbance measurement, in which a spectrophotometer has been used.11 A typical measurement platform for colorimetric sensors using AuNPs is a spectrophotometer.11 The polychromic light that penetrates through the sample can be scanned over a specific wavelength range. A primary drawback of this method is a significant signal loss due to light scattering in the presence of AuNPs (particularly, at high AuNP concentrations). Thus, only a low range of AuNP concentrations could be used in the previous studies, resulting in a relatively high limit of detection (LOD) (25 nM).4
These limitations of absorbance-based detection can be overcome by employing reflectance configurations. Unlike the absorbance-based aptasensors in which the absorbance of AuNPs is analyzed indirectly from the spectrum transmitted, the reflectance-based measurement is a direct measurement of the reflected spectrum from the surface plasmon resonance of AuNPs (Fig. 1). In this approach, high AuNP concentrations are actually more desirable, since they can amplify the signal, potentially improving the sensitivity and LOD. In addition, since the reflectance-based measurement can be configured with flexible optical fibers, the system can be constructed on diverse platforms such as flow cells and microfluidic channels.2,12,13 However, there is also a challenge in reflectance-based measurements. The challenge arises from the fact that there are two types of reflections: specular reflection at the surface of the solution with no transmission into the solution and diffuse reflection in which the radiation penetrates into the solution and is reflected at the surface of particles with partial absorbance and scattering.14 When the measurement of diffuse reflection from the particles suspended in a solution is desired, the specular reflection from the solution or container surface must be minimized. This would require optimization of the incident angle and the sample container. So far, few studies have been reported using reflectance configurations as biosensors. A gold nanoparticle thin film modified with biotin was reported for monitoring biotin–avidin interactions and they used a fixed incident angle.10 There have been no reports using any reflectance configurations together with aptamers and AuNPs.
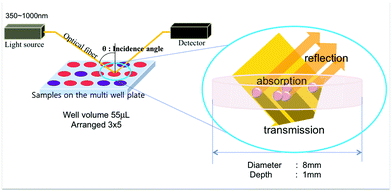 | ||
| Fig. 1 The scheme of the reflectance-based aptasensor and the multi-well plate and the colorimetric aptasensor using AuNPs. | ||
This study presents, for the first time, a novel reflectance-based colorimetric aptasensor using gold nanoparticles for the detection of oxytetracycline. It was found that the reflectance-based measurement at two wavelengths (650 and 520 nm) can generate more stable and sensitive signals than the absorbance-based sensors to determine the aggregation of AuNPs, even at high AuNP concentrations. OTC was detected at concentrations as low as 1 nM in both buffer solution and tap water, which was 25-fold more sensitive, compared to the previous absorbance-based colorimetric aptasensors. Moreover, the sample does not need to be contained in the standard cuvettes and thus the sample loading platform can be miniaturized into portable and on-site diagnosis sensors. This novel reflectance-based aptasensor is believed to address the drawbacks of the absorbance-based sensors and thus has great potential to be developed into a portable sensor system for on-site diagnosis applications.
Materials and methods
Materials and DNA aptamers
All the chemicals were purchased from Sigma-Aldrich, unless specified otherwise. An OTC binding ssDNA aptamer (OBA) (5′-CGTACGGAATTCGCTAGCACGTTGACGCTGGTGCCCGGTTGTGGTGCGAGTGTTGTGTGGATCCGAGCTCCACGTG-3′) was synthesized by Genotech Inc. (Korea) and sorted after dissolving in distilled water.15 AuNPs were synthesized by citrate reduction of HAuCl4 as reported previously.16Experimental set-up for reflectance measurement
The experimental set-up consisted of a light source (HL-2000-FHSA, Ocean Optics), a spectrometer (USB4000, Ocean Optics), and optical fibers (all from Ocean Optics, Dunedin, FL). Optical fibers were fixed in a holder to control the angle between the optical fibers and the well plate that contains the sample. Multi-well plates used were made of acrylic plastic by machining. We custom designed wide but shallow (8 mm in diameter and 1 mm in depth) 15-multiwell plates arranged in a 3 × 5 format for our study. The wide wells were designed to obtain strong reflectance intensity. In order to decrease the required sample volume, we designed shallow wells. The internal volume of the well was approximately 50 μl. The well size was also designed to be slightly larger than the diameter of the projected light (3–6 mm). The USB4000 measured light spectrum reflected by AuNPs and the aggregation of AuNPs was analyzed by using the ratio of reflectance intensities at 520 nm and 650 nm wavelengths. All devices were set up on a probe station and remained horizontal except for the optical fiber.Process optimization study and assay protocol
All the experiments were conducted by using the same amount of solutions: 51.3 μl of AuNP compounds, 2.85 μl of ssDNA aptamer, 2.85 μl of target compounds, and 3 μl of NaCl solution. The OTC target was dissolved in binding buffer (100 mM NaCl, 2 mM MgCl2·6H2O, 5 mM KCl, 1 mM CaCl2, 20 mM C4H11NO3). The total volume of the solution introduced into individual wells for optical analysis was 60 μl. At first, AuNPs were mixed with ssDNA aptamers by shaking at 200 rpm for 30 min. Then the target compounds were pipetted into the mixture of AuNPs and ssDNA aptamers. After another 30 min of mixing, 1 μl of NaCl (0–1.5 M) was injected 3 times because 3 μl of NaCl injection at a time can cause excessive electrostatic instability. The target compound, oxytetracycline, was replaced with a buffer solution in all the optimization processes. The target concentrations tested in the binding assay were 1 nM to 25 μM in both buffer and tap water.Results and discussion
Optimization of reflectance-based colorimetric aptasensors
In the reflectance-based aptasensor using unmodified AuNPs, the optimization of the incidence and reflection angles is critical because the portion of light reflected from the AuNP surface varies with the incidence angle. In our experiment, the diffuse reflection at the surface of AuNPs is of primary interest and thus the incidence angle and the container were carefully selected through experimental characterization in order to minimize the specular reflection. Though the incidence angle is an important factor in reflectance-based approaches, the effect of the incidence angle has been disregarded in previous research. Other critical factors that influence the performance of this type of aptasensor using umAuNPs significantly are salt concentrations, the ratio of AuNPs to aptamer, and AuNP concentrations. These parameters were optimized through experimental characterization as well.Effect of incidence angle
We designed the reflectance-based aptasensor where the incidence angle was able to be changed. As the incidence angle changed, the portion of the reflected light also changed. The light can be reflected from the surface of the solution and AuNPs. To increase the portion of the reflected light on the AuNP surface, we performed the measurement of samples at 20°, 30°, 40° and 50° incidence angles from the optical fibers (Fig. 2). While the peak shifting from 520 nm to 650 nm was observed for all the four angles investigated as AuNP aggregation occurred, the reflectance peak at 520 nm wavelength was the sharpest at 50° incidence angle, and the ratio R650/R520 was the lowest. It means that the amount of the reflected light on the AuNP surface was the highest at 50° angle. We performed all other experiments at this optimal incidence angle.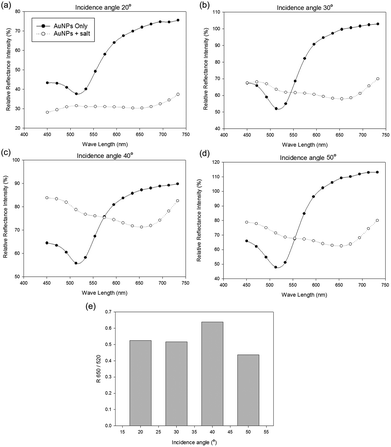 | ||
| Fig. 2 Relative reflectance intensity at 520 and 650 nm light wavelengths for different incidence angles: (a) 20°, (b) 30°, (c) 40°, (d) 50°, (e) R650/R520 of AuNPs at different incidence angles. | ||
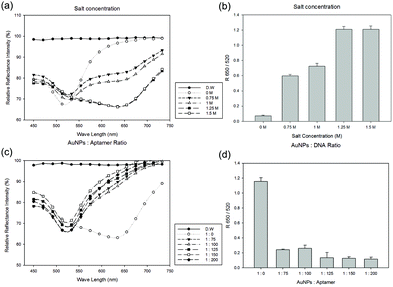
Effects of salt concentration and the AuNP to aptamer ratio
There are three important parameters influencing the performance of this reflectance-based colorimetric aptasensor, such as AuNP concentration, salt concentrations, and the ratio of AuNPs to aptamers. These parameters are interdependent on each other, and therefore they should be optimized in the right order. First, we fixed the AuNP concentration at 2.5 nM and optimized the salt concentration. When the AuNPs aggregated, the reflectance peak shifted from 520 nm to 650 nm wavelength in the spectra and the ratio of R650/R520 increased (Fig. 3(a) and (b)). These color changes and peak shifts were saturated at a specific salt concentration (∼1.25 M), as shown in Fig. 2a and b, which is considered to be optimal.The ssDNA aptamers can be adsorbed on the AuNP surface by the electrostatic forces. The aptamers attached on the AuNP surface make a stable state, even at high salt concentrations. The ratio of AuNPs to aptamers is also a key parameter in this step. For finding an appropriate AuNP to aptamer ratio, we performed experiments using the ratios 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, 1
0, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75, 1
75, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100, 1
100, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 125, 1
125, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 150 and 1
150 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200. As the aptamer concentration increased, the AuNPs became more stable (Fig. 3(c) and (d)). Therefore, the optimum ratio of AuNPs to aptamers was determined to be 1
200. As the aptamer concentration increased, the AuNPs became more stable (Fig. 3(c) and (d)). Therefore, the optimum ratio of AuNPs to aptamers was determined to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 125.
125.
Assessment of specificity for OTC
Oxytetracycline is a tetracycline group antibiotic. Oxytetracycline, tetracycline and doxycycline have similar structures, except just one functional group. It has already been reported that the specificity of the OTC binding aptamer is good,3 and the specificity of this reflectance-based aptasensor configuration was confirmed again for 50 μM of tetracycline, doxycycline and diclofenac, respectively. In fact, in spite of the similar structures of tetracyclines, the peak shift occurred only with oxytetracycline, not with other similar tetracyclines (Fig. 4).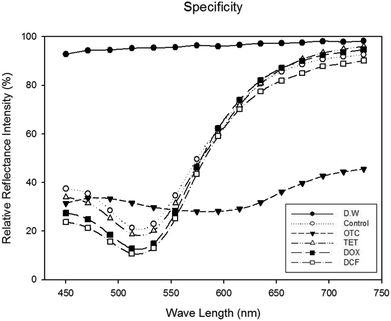 | ||
| Fig. 4 Specificity of the reflectance-based colorimetric aptasensor. The final concentration of oxytetracycline, tetracycline, doxycycline, and diclofenac is 50 μM. | ||
Effect of AuNP concentrations and dose-dependent assay
We performed a dose dependent binding assay at different AuNP concentrations for optimizing the AuNP concentration and measuring the limit of detection (LOD) (Fig. 5). In our previous work based on an absorbance system, the optimal AuNP concentration was 0.2 nM and the LOD was 25 nM.4,17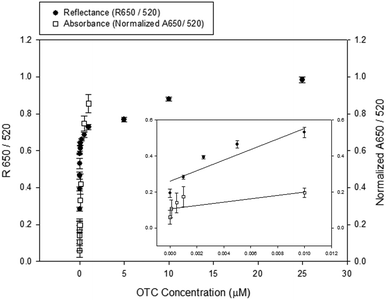 | ||
| Fig. 5 An overlay plot showing a dose-dependence of this reflectance-based aptasensor and the absorbance-based aptasensor using unmodified AuNPs for the detection of oxytetracycline. Filled circles indicated reflectance data while empty squares indicated absorbance data published in our previous study.17 The left vertical axis represents R650/R520 for the reflectance intensity ratio and the right vertical axis represents the normalized A650/A520 for the absorbance intensity ratio. | ||
In this reflectance-based method, the reflectance light from AuNPs, not scattering light, at a certain angle is measured. So, higher the AuNP concentrations, stronger the reflectance light obtained. Therefore, in this study we have used higher AuNP concentrations than that used in other methods such as absorbance-based colorimetry, in which the absorbance signal is more decreased at higher AuNP concentrations. In this novel reflectance-based aptasensor system, better results were obtained at higher AuNP concentrations even if the same aptamer sequence was used. With an AuNP concentration of 10 nM, the LOD was 1 nM, which is 25-fold smaller than the previous result obtained by the absorbance system. In addition, even though the AuNP concentration (10 nM) in this novel reflectance-based method was higher than the previous method (0.2–2 nM), the amount of sample required was decreased about 0.75 times because the experiments were conducted with only 60 μl solution.
In order to be used for on-site applications, this sensor should be functional in any sample solutions. The detection of oxytetracycline was attempted in a tap water solution for this purpose (Fig. 6). The OTC was dissolved in tap water at different concentrations. All other conditions were the same as the binding assay described in Fig. 3. Even in tap water, the LOD remained as low (1 nM) as in the buffer solution.
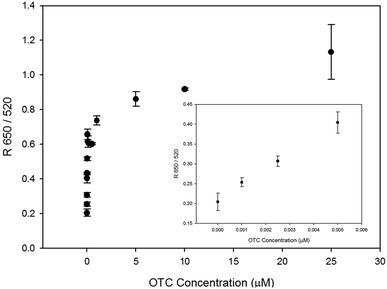 | ||
| Fig. 6 Dose-dependent measurement of oxytetracycline using this reflectance-based aptasensor in tap water. | ||
Table 1 shows the comparison of the current reflectance-based aptasensor with absorbance-based aptasensors and other biosensors (cantilever sensors, light scattering agglutination assay, and indirect competitive assay) with regard to the sensor performance for the detection of OTC. LOD, the limit of quantification (LOQ), linear dynamic range, and EC50 values of the sensors were obtained from the literature.2,4,18,19 The linear range of the reflectance-based aptasensor was 0–10 nM. The reflectance-based aptasensor shows lower LOD/LOQ and does not require high sample volume or pre-treatment. Therefore, it seems to be suitable for on-site analysis of low concentration targets such as OTC. The more complicated food sample tests could be a subject of further study.
| Pros | Cons | LOD/LOQ | Dynamic range | EC50 | Ref. | |
|---|---|---|---|---|---|---|
| Reflectance-based aptasensor | Low LOD | 1 nM/4 nM | 1 nM–1 μM | 188 nM | This study | |
| Low sample vol. | ||||||
| No pre-treatment | ||||||
| Absorbance-based aptasensor | No pre-treatment | High LOD | 25 nM/— | 0.025–1 μM | 313 nM | 4 |
| High sample vol. | ||||||
| Cantilever sensor | Low LOD | Pre-treatment | 0.2 nM/— | 1–100 nM | 30 nM | 18 |
| Long measuring time | ||||||
| Light scattering agglutination assay | Real-time monitoring | High LOD | 217 nM/— | 0.217–21.7 μM | — | 2 |
| Indirect competitive assay | High recovery rate in spiked milk | High LOD | 27 nM/ 108 nM | — | — | 19 |
| Pre-treatment |
Conclusions
In this study, we developed a novel reflectance-based aptasensor using unmodified gold nanoparticles for the detection of oxytetracycline. This reflectance aptasensor can measure the peak shift of the localized surface plasmon resonance as the AuNPs aggregate in the solution state. Compared with the absorbance-based sensors previously reported, this reflectance-based system has significant advantages. First, it can measure the reflectance from the AuNP surface directly, so reflectance signals increase at high AuNP concentrations. As a result, the sensitivity of the aptasensor increased 25-fold. Second, we may be able to use different loading platforms with various shapes, such as well plates, microfluidic channels and disc plates. The reflectance method offers more flexibility in terms of system construction compared with the absorbance method. Third, small amounts of sample and reagents are necessary for detection. We believe that our novel reflectance-based aptasensor has great potential to be developed into a portable sensor system for on-site diagnosis applications.Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) Grant funded by the Korea Government (MEST) (no. 2013R1A1A2021531).Notes and references
- F. K. Muriuki, W. O. Ogara, F. M. Njeruh and E. S. Mitema, J. Vet. Sci., 2001, 2, 97–101 CAS.
- K. Kim, M.-B. Gu, D.-H. Kang, J.-W. Park, I.-H. Song, H.-S. Jung and K.-Y. Suh, Electrophoresis, 2010, 31, 3115–3120 CrossRef CAS PubMed.
- Y.-J. Kim, Y. Kim, J. Niazi and M. Gu, Bioprocess Biosyst. Eng., 2010, 33, 31–37 CrossRef CAS PubMed.
- Y. S. Kim, J. H. Kim, I. A. Kim, S. J. Lee, J. Jurng and M. B. Gu, Biosens. Bioelectron., 2010, 26, 1644–1649 CrossRef CAS PubMed.
- Y. S. Kim, J. H. Niazi and M. B. Gu, Anal. Chim. Acta, 2009, 634, 250–254 CrossRef CAS.
- R. Elghanian, J. J. Storhoff, R. C. Mucic, R. L. Letsinger and C. A. Mirkin, Science, 1997, 277, 1078–1081 CrossRef CAS.
- J.-S. Lee, M. S. Han and C. A. Mirkin, Angew. Chem., Int. Ed., 2007, 119, 4171–4174 CrossRef PubMed.
- F. Xia, X. Zuo, R. Yang, Y. Xiao, D. Kang, A. Vallée-Bélisle, X. Gong, J. D. Yuen, B. B. Y. Hsu, A. J. Heeger and K. W. Plaxco, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 10837–10841 CrossRef CAS PubMed.
- S. K. Ghosh and T. Pal, Chem. Rev., 2007, 107, 4797–4862 CrossRef CAS PubMed.
- H. M. Hiep, H. Yoshikawa, M. Saito and E. Tamiya, ACS Nano, 2009, 3, 446–452 CrossRef CAS.
- Y. S. Kim, J. H. Kim, I. A. Kim, S. J. Lee and M. B. Gu, Biosens. Bioelectron., 2011, 26, 4058–4063 CrossRef CAS PubMed.
- M. Ahmad and R. Narayanaswamy, Anal. Chim. Acta, 1994, 291, 255–260 CrossRef CAS.
- N. A. Yusof and M. Ahmad, Sens. Actuators, B, 2003, 94, 201–209 CrossRef CAS.
- R. Narayanaswamy, Analyst, 1993, 118, 317–322 RSC.
- J. H. Niazi, S. J. Lee, Y. S. Kim and M. B. Gu, Bioorg. Med. Chem., 2008, 16, 1254–1261 CrossRef CAS.
- J.-e. Lee, J. Kim, S. Lee, J. Kim, S. Mah and M. Gu, BioChip J., 2013, 7, 180–187 CrossRef CAS.
- Y. S. Kwon, N. H. Ahmad Raston and M. B. Gu, Chem. Commun., 2014, 50, 40–42 RSC.
- H. Hou, X. J. Bai, C. Y. Xing, N. Y. Gu, B. L. Zhang and J. L. Tang, Anal. Chem., 2013, 85, 2010–2014 CrossRef CAS PubMed.
- C. H. Kim, L. P. Lee, J. R. Min, M. W. Lim and S. H. Jeong, Biosens. Bioelectron., 2014, 51, 426–430 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Fig. S1 and S2. See DOI: 10.1039/c5an00726g |
| This journal is © The Royal Society of Chemistry 2015 |