Open Access Article
Open Access ArticleKey steps towards the oriented immobilization of antibodies using boronic acids
Florine
Duval
a,
Teris A.
van Beek
*a and
Han
Zuilhof
ab
aLaboratory of Organic Chemistry, Wageningen University, Dreijenplein 8, 6703 HB Wageningen, The Netherlands. E-mail: teris.vanbeek@wur.nl; Fax: +31 317 484914; Tel: +31 317 482361
bDepartment of Chemical and Materials Engineering, King Abdulaziz University, Jeddah, Saudi Arabia
First published on 10th August 2015
Abstract
Oriented immobilization of antibodies using boronic acids shows a strong potential for improving immunoassay performance but is not yet widely used, possibly because of the difficulties encountered in its implementation. How to choose the boronic acid structure and how should it be attached to the surface? How to choose an antibody that will bind to the boronic acid? Under which conditions should the binding take place for an effective oriented antibody immobilization? How to make sure that the antibody stays on the surface? This tutorial review provides answers to these questions through analysis of the literature and personal suggestions, and thereby intends to facilitate the development of this promising antibody immobilization strategy.
1 Introduction
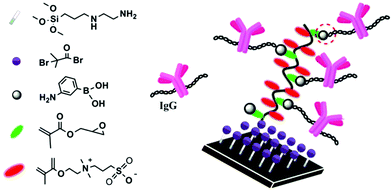
Antibody (Ab) immobilization is an important step in the preparation of devices for the detection of various analytes (= antigens) such as proteins, bacteria, viruses, drugs and toxins. Antibodies (Abs) are proteins, and protein immobilization strategies, which have been reviewed in detail,1,2 can be divided into two categories: random and oriented (or site-specific). Oriented immobilization is especially important for Abs in immunosensing applications, as their binding sites need to be kept available for antigen binding. Using oriented immobilization, signal enhancement factors of over 200 have been obtained,3 showing the significance of Ab orientation. Strategies for oriented Ab immobilization, which have recently been reviewed,4,5 aim at attaching the Ab to the surface via its Fc region (see the Ab structure in Fig. 1), far away from the binding sites. Abs are N-glycosylated (i.e., carbohydrate chains are attached to the amide nitrogen of Asn residues of Abs and thus defined as N-glycans) in their Fc regions, thus a proper orientation of the antibody can be achieved by immobilization via its N-glycans. This has mainly been done in two ways, both taking advantage of available vicinal diols in the N-glycans: (1) by oxidation of diols to aldehydes, which then react with nucleophiles attached to the surface, and (2) by reaction of diols with boronic acids attached to the surface. The present review focuses on the second approach.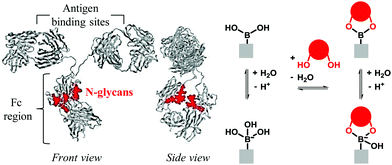 | ||
| Fig. 1 Principle of oriented antibody immobilization using boronic acids. Left: crystal structure of immunoglobulin with highlighted N-glycans (3D model from NCBI, recolored); right: scheme of the boronic acid–diol complexation.19 | ||
Boronic acids react with 1,2-diols to form boronate esters (Fig. 1). This reaction has been used for many applications, including the separation and immobilization of glycoproteins (including antibodies).6 Oriented Ab immobilization using boronic acids (through the same reaction) was reported for the first time in 2009 by Lin et al.,7 and has appeared in another eleven papers since then.8–18 An overview of these articles is given in Table 1. This immobilization method appears very attractive, as it presents several advantages over more common ones: it leaves the binding sites available for antigen binding (oriented vs. random immobilization), it can be done in one step without prior antibody modification (vs. oxidation with periodate, use of antibody fragments or enzymatic modification of the N-glycans), and it is relatively economical (vs. Fc-chain binding proteins such as protein A/G). Moreover, several comparative studies (in Ab-BA 1, 2, 7 and 11) suggested that the highest antigen detection sensitivity was achieved when Abs were immobilized using boronic acids.
| Paper code | Main feature | Ref. |
|---|---|---|
| Ab-BA 1 | First Ab immobilization via BA | 7 |
| Ab-BA 2 | Novel immunosensing application | 8 |
| Ab-BA 3 | Novel immunosensing application | 9 |
| Ab-BA 4 | Novel immunosensing application | 10 |
| Ab-BA 5 | Novel immunosensing application | 11 |
| Ab-BA 6 | BA on zwitterionic polymer brushes | 12 |
| Ab-BA 7 | Probing of the available binding sites | 13 |
| Ab-BA 8 | Direct assessment of Ab orientation | 14 |
| Ab-BA 9 | Microarray of 71 Abs | 15 |
| Ab-BA 10 | First irreversible Ab immob. via BA | 16 |
| Ab-BA 11 | Novel immunosensing application | 17 |
| Ab-BA 12 | Novel immunosensing application | 18 |
The number of papers reporting this immobilization strategy, however, remains limited (12 papers, four of them having been reviewed by Wang et al.6). So the question arises: where does its lack of popularity come from? We believe that many researchers may have tested Ab immobilization via boronic acids, that likely problems were encountered in most cases, and that therefore few successful studies were published. Although promising, this immobilization strategy is not as simple as it seems. In this tutorial review, the reader is guided through several points that need to be considered to allow the development of the full potential of this still very appealing approach.
2 Step-wise guide
2.1 Preparation of boronic acid-functionalized surface
The first step towards a successful Ab immobilization via boronic acids is a surface modification with the right molecules.Furthermore, an antifouling coating helps to minimize other types of unwanted (e.g., electrostatic or hydrophobic) interactions between Ab and the surface. PEG is widely used for its antifouling properties, and zwitterionic polymer brushes prevent the nonspecific adsorption of biomolecules even more effectively.21,22 Thus, it may be a good idea to prepare boronic acid-containing zwitterionic polymer brushes, as was done in Ab-BA 6 (Fig. 2). Alternatively, commercially available substrates with antifouling coatings decorated with reactive groups such as NHS or epoxide may be modified with a boronic acid that contains an appropriate reactive group (such as 3-aminophenylboronic acid). This has for example been done in Ab-BA 10, in which commercial PEG–NHS slides were used. One has to keep in mind, however, that some antifouling polymers bind with boronic acid groups, possibly resulting in a decreased availability of the boronic acid groups for subsequent Ab binding. This problem may be encountered with dextran, which has been used as a boronic acid blocking agent in Ab-BA 1, 6 and 10, and with polymers of unknown structure, based on our own experience with proprietary coatings of some commercial antifouling slides.
2.2 Choice of antibody with suitable glycosylation
Many different Abs were used in the Ab-BA papers. Few of them were fully described and the rationale behind their selection was never explained. Considering the intrinsic properties of an Ab, a key factor for its successful immobilization is the composition of the N-glycans at its Fc chain. These N-glycans must present diols that have a suitable conformation for binding with boronic acids. Many monosaccharides bind with boronic acids, but when they are attached to each other, relatively weak interactions between boronic acids and the formed glycans are enabled. Sialic acid, however, has a side chain with a free and flexible glycol function that may bind more strongly to boronic acids.19 Based on this, the presence of sialic acids in the Ab N-glycans is highly favorable, and possibly even required for a successful immobilization. The Ab N-glycans vary with the cell line, animal species, culture conditions and even from batch to batch, resulting in the presence or absence of sialic acids at the glycan chain ends.29Analysis of Ab candidates for immobilization is therefore advisable. Ab glycosylation may be analyzed using mass spectrometric and chromatographic techniques,30 or more simply, Abs may be screened for sialic acids using the periodic acid-Schiff method31 (we used a commercial glycoprotein carbohydrate estimation kit). The affinity of Abs for boronic acids may also directly be assessed by boronate affinity chromatography using a commercially available boronate column,32,33 although the results will also depend on the properties of the column and the binding conditions (see section 2.3).
2.3 Antibody immobilization on the boronic acid-functionalized surface
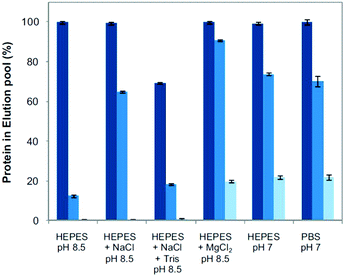 | ||
| Fig. 3 Binding of proteins to boronic acids as displayed by percentage of bound and then eluted IgG (dark), albumin (middle), and insulin (light) in different adsorption buffers: (i) 20 mM HEPES at pH 8.5; (ii) 20 mM HEPES, 150 mM NaCl at pH 8.5; (iii) 20 mM HEPES, 150 mM NaCl, 15 mM Tris at pH 8.5; (iv) 20 mM HEPES, 150 mM MgCl2 at pH 8.5; (v) 20 mM HEPES at pH 7; and (vi) 10 mM phosphate, 150 mM NaCl at pH 7. Reproduced with permission.33 | ||
2.4 Stability
The reaction between a boronic acid and a diol to form a boronate ester is reversible, which may strongly disturb Ab immobilization. The diol content and pH of the solution both have a strong influence on the equilibrium.If the antigen is a protein, then chances are high that it is glycosylated.34 Therefore, it may expel the Ab from the surface, resulting in erroneous measurements. Blocking antigen diols with 3-aminophenylboronic acid may not only cause the problem described in the previous paragraph, but also cause blocking of the antigen epitopes or nonspecific interactions between the antigen and the surface. However, if the antigen is big enough and the Ab layer is dense enough, then steric hindrance may prevent the antigen from accessing the boronic acid.35 If steric hindrance is not sufficient, then we recommend a synthetically involved but elegant solution: make the boronic acid-mediated immobilization irreversible (see below).
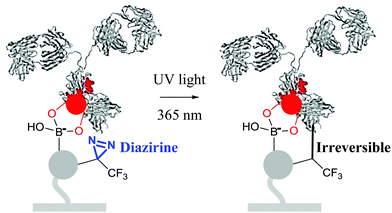 | ||
| Fig. 4 Strategy for oriented and irreversible antibody immobilization.16 | ||
3 Conclusion and outlook
Ab immobilization using boronic acids requires substantial preliminary work: preparing a boronic acid-containing antifouling surface (most likely via several chemical steps), finding an Ab with suitable N-glycans, finding binding conditions that result in effective and oriented Ab immobilization, and making sure that the immobilized Ab is not expelled from the surface by competing diols or harsh regeneration conditions.However, once the basic requirements are met and initial optimizations are done, Ab immobilization via boronic acids may result in a much higher sensitivity for antigen detection than what can be achieved by random immobilization, and an improvement of cost and simplicity compared to other strategies for oriented Ab immobilization.
Future research in this field may give precious information about trends in the glycosylation of Abs and their affinity for boronic acids, and explore the effect of boronic acid structure, surface architecture and binding conditions on Ab immobilization and orientation. This could lead to further strategies for oriented and irreversible Ab immobilization, making Ab immobilization via boronic acids a well-established method within the fabrication of high-performance biosensors. Moreover, further knowledge about the interactions between boronic acids and antibodies may lead to improvements in other related fields, such as antibody, or more generally, glycoprotein purification and site-specific antibody labelling.
Notes and references
- F. Rusmini, Z. Zhong and J. Feijen, Biomacromolecules, 2007, 8, 1775–1789 CrossRef CAS PubMed.
- P. Jonkheijm, D. Weinrich, H. Schroder, C. M. Niemeyer and H. Waldmann, Angew. Chem., Int. Ed., 2008, 47, 9618–9647 CrossRef CAS PubMed.
- A. K. Trilling, M. M. Harmsen, V. J. B. Ruigrok, H. Zuilhof and J. Beekwilder, Biosens. Bioelectron., 2013, 40, 219–226 CrossRef CAS PubMed.
- A. K. Trilling, J. Beekwilder and H. Zuilhof, Analyst, 2013, 138, 1619–1627 RSC.
- A. Makaraviciute and A. Ramanaviciene, Biosens. Bioelectron., 2013, 50, 460–471 CrossRef CAS PubMed.
- X. Wang, N. Xia and L. Liu, Int. J. Mol. Sci., 2013, 14, 20890–20912 CrossRef PubMed.
- P. C. Lin, S. H. Chen, K. Y. Wang, M. L. Chen, A. K. Adak, J. R. R. Hwu, Y. J. Chen and C. C. Lin, Anal. Chem., 2009, 81, 8774–8782 CrossRef CAS PubMed.
- J. A. A. Ho, W. L. Hsu, W. C. Liao, J. K. Chiu, M. L. Chen, H. C. Chang and C. C. Li, Biosens. Bioelectron., 2010, 26, 1021–1027 CrossRef CAS PubMed.
- M. Moreno-Guzmán, I. Ojeda, R. Villalonga, A. González-Cortés, P. Yáñez-Sedeño and J. M. Pingarrón, Biosens. Bioelectron., 2012, 35, 82–86 CrossRef PubMed.
- M. Moreno-Guzmán, A. González-Cortés, P. Yáñez-Sedeño and J. M. Pingarrón, Electroanalysis, 2012, 24, 1100–1108 CrossRef PubMed.
- Z. Wang, K. Shang, J. Dong, Z. Cheng and S. Ai, Microchim. Acta, 2012, 179, 227–234 CrossRef CAS.
- L. Song, J. Zhao, S. Luan, J. Ma, J. Liu, X. Xu and J. Yin, ACS Appl. Mater. Interfaces, 2013, 5, 13207–13215 CAS.
- W. C. Liao and J. A. Annie Ho, Biosens. Bioelectron., 2014, 55, 32–38 CrossRef CAS PubMed.
- H. Y. Song, J. Hobley, X. Su and X. Zhou, Plasmonics, 2014, 9, 851–858 CrossRef CAS.
- M. González-González, R. Bartolome, R. Jara-Acevedo, J. Casado-Vela, N. Dasilva, S. Matarraz, J. García, J. A. Alcazar, J. M. Sayagues, A. Orfao and M. Fuentes, Anal. Biochem., 2014, 450, 37–45 CrossRef PubMed.
- A. K. Adak, B.-Y. Li, L.-D. Huang, T.-W. Lin, T.-C. Chang, K. C. Hwang and C.-C. Lin, ACS Appl. Mater. Interfaces, 2014, 6, 10452–10460 CAS.
- L.-D. Huang, A. K. Adak, C.-C. Yu, W.-C. Hsiao, H.-J. Lin, M.-L. Chen and C.-C. Lin, Chem. – Eur. J., 2015, 21, 3956–3967 CrossRef CAS PubMed.
- S. A. Lim and M. U. Ahmed, Biosens. Bioelectron., 2015, 70, 48–53 CrossRef CAS PubMed.
- J. A. Peters, Coord. Chem. Rev., 2014, 268, 1–22 CrossRef CAS PubMed.
- H. Li and Z. Liu, TrAC, Trends Anal. Chem., 2012, 37, 148–161 CrossRef CAS PubMed.
- C. Rodriguez Emmenegger, E. Brynda, T. Riedel, Z. Sedlakova, M. Houska and A. B. Alles, Langmuir, 2009, 25, 6328–6333 CrossRef CAS PubMed.
- S. Joshi, P. Pellacani, T. A. van Beek, H. Zuilhof and M. W. F. Nielen, Sens. Actuators, B, 2015, 209, 505–514 CrossRef CAS PubMed.
- F. Duval, T. A. Van Beek and H. Zuilhof, Synlett, 2012, 1751–1754 CAS.
- D. Zhang, K. L. Thompson, R. Pelton and S. P. Armes, Langmuir, 2010, 26, 17237–17241 CrossRef CAS PubMed.
- A. E. Ivanov, A. Kumar, S. Nilsang, M. R. Aguilar, L. I. Mikhalovska, I. N. Savina, L. Nilsson, I. G. Scheblykin, M. V. Kuzimenkova and I. Y. Galaev, Colloids Surf., B, 2010, 75, 510–519 CrossRef CAS PubMed.
- P.-C. Chen, L.-S. Wan, B.-B. Ke and Z.-K. Xu, Langmuir, 2011, 27, 12597–12605 CrossRef CAS PubMed.
- R. Pelton, Y. Cui, D. Zhang, Y. Chen, K. L. Thompson, S. P. Armes and M. A. Brook, Langmuir, 2012, 29, 594–598 CrossRef PubMed.
- Y.-W. Lu, C.-W. Chien, P.-C. Lin, L.-D. Huang, C.-Y. Chen, S.-W. Wu, C.-L. Han, K.-H. Khoo, C.-C. Lin and Y.-J. Chen, Anal. Chem., 2013, 85, 8268–8276 CrossRef CAS PubMed.
- S. Raju, BioProcess Int., 2003, 1, 44–53 Search PubMed.
- C. Huhn, M. H. J. Selman, L. R. Ruhaak, A. M. Deelder and M. Wuhrer, Proteomics, 2009, 9, 882–913 CrossRef CAS PubMed.
- G. P. Roberts, Histochem. J., 1977, 9, 97–102 CrossRef CAS.
- B. Zhang, S. Mathewson and H. Chen, J. Chromatogr., A, 2009, 1216, 5676–5686 CrossRef CAS PubMed.
- A. M. Azevedo, A. G. Gomes, L. Borlido, I. F. S. Santos, D. M. F. Prazeres and M. R. Aires-Barros, J. Mol. Recognit., 2010, 23, 569–576 CrossRef CAS PubMed.
- G. A. Khoury, R. C. Baliban and C. A. Floudas, Sci. Rep., 2014, 1, 90 Search PubMed.
- S. Liu, X. Zhang, Y. Wu, Y. Tu and L. He, Clin. Chim. Acta, 2008, 395, 51–56 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |