Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Controlled growth of immunogold for amplified optical detection of aflatoxin B1
Xu
Wang
,
Reinhard
Niessner
and
Dietmar
Knopp
*
Institute of Hydrochemistry, Chair for Analytical Chemistry, Technische Universität München, Marchioninistrasse 17, D-81377 München, Germany. E-mail: dietmar.knopp@ch.tum.de; Fax: +49-89-2180-78255; Tel: +49-89-2180-78252
First published on 12th January 2015
Abstract
A simple, sensitive and cost-effective method for the analysis of the mycotoxin aflatoxin B1 (AFB1) has been established based on controlled growth of immunogold. AFB1-BSA conjugate modified magnetic beads were employed as capture probe and anti-AFB1 antibody-coated gold colloids were used as detection probe for the immunological recognition of AFB1, as well as for signal transduction. The immune recognition event is converted into the gold enlargement signal which can be quantitatively measured by UV-vis spectroscopy. The autocatalytic enlargement of immunogold was conducted in aqueous solution containing chloroauric acid, hexadecyltrimethylammonium bromide and ascorbic acid. The reaction could be stopped by the addition of sodium thiosulfate. The final absorbance and resonance light scattering intensity were highly dependent on immunogold concentration. After gold enhancement, the sensitivity of the immunoassay was improved and total assay time reduced to 1 h. Under optimized conditions, the linear range and lower detection limit was 0.01–1 ng mL−1 and 7 pg mL−1, respectively. The proposed method offers great promise for sensitive detection of other mycotoxins and organic pollutants.
Introduction
Gold nanoparticles (AuNPs) have attracted great attention in the bioanalytical field owing to their unique physical and chemical properties, such as easy preparation, simplicity of modification, superior compatibility and excellent optical property.1,2 They are frequently employed as labels for different biological receptors, including enzymes, antibodies, aptamers/DNA, and other biomolecules,3,4 which have been utilized for the detection of a wide variety of analytes.5,6Based on the unique distance-dependent surface plasmon resonance (SPR) property of AuNPs, numerous optical probes have been developed.7,8 In addition, AuNPs have also been extensively used for tag amplification.9 For instance, owing to the large surface area of AuNPs, active biomolecules like enzymes,10,11 artificial DNAzymes12 and enzyme-labeled antibodies13,14 were immobilized on gold surface, which can efficiently catalyze the specific substrates to generate a distinguishable signal change, thereby achieving the enzymatic signal amplification. Further, signal amplification was realized by utilizing the special catalytic properties of AuNPs. For example, AuNPs can catalyze the decoloration of organic dyes like methyl orange,15 4-nitrophenol16 and methylene blue,17 resulting in obvious absorbance change, which can be easily read out with the naked eye or special analytical equipment. As another example, AuNPs served as seeds for silver enlargement where the reduction and deposition of silver ions are catalyzed by the AuNPs.18
The so-called silver staining method has been well developed and extensively applied in biomolecular detection.19 However, as a disadvantage, the silver salt is usually sensitive to pH, phosphates, chloride ions and natural light, which limits its practical application. As an alternative, the gold staining, i.e., using gold salt for catalytic enlargement of AuNPs, has solved these problems. The gold staining methods have been widely used for the detection of various targets such as proteins and DNA.20–22 However, this technique is mostly applied onto solid substrates like glass slides23 and nitrocellulose strips,20 therefore, strict control of the reaction conditions are required to obtain acceptable reproducibility. Several homogeneous detection formats have been developed to solve this problem.24–26 The AuNPs were enlarged in aqueous solution, which shows some attractive attributes such as simpleness, low cost and high sensitivity. But generally, a long reaction time is required for homogeneous gold nanogrowth.26 Since the gold enlargement is time-dependent, the reaction continues until all the gold ions in growth solution are exhausted. Significantly, we recently found that sodium thiosulfate can efficiently stop the reaction, which makes the homogenous gold staining more controllable and convenient.
In this work, controlled growth of AuNPs in aqueous solution was studied in-depth by UV-vis spectroscopy and resonance light scattering (RLS) technique, using hexadecyltrimethylammonium bromide (CTAB) as stabilizing surfactant and ascorbic acid (AA) as reducing agent. The controlled gold staining was then used for signal amplification in competitive immunoassay (Fig. 1). The mycotoxin aflatoxin B1 (AFB1) was chosen as the model analyte.27 Although various methods have been developed for AFB1 detection, such as instrumental analysis using high-performance liquid chromatography (HPLC)28 or liquid chromatography coupled to mass spectrometry (LC-MS)29 and different immunological assays,30–32 even more simple and rapid methods are still desirable. AFB1-BSA conjugate modified magnetic beads (AFB1-BSA-Fe3O4, MBs) were employed as capture probe, while anti-AFB1 antibody-coated AuNPs (Ab-AuNPs) were used as detection probe for immunological recognition of AFB1, as well as for signal transduction. Since MBs were removed from the reaction mixture, they did not participate in the subsequent gold enlargement. After signal amplification, the sensitivity of the immunoassay increased distinctly. To the best of our knowledge, this is the first time that homogeneous gold staining was applied to a competitive immunoassay.
 | ||
| Fig. 1 Schematic illustration of the homogeneous gold staining for amplified optical detection of AFB1. | ||
Experimental
Materials
Chloroauric acid (HAuCl4), hexadecyltrimethylammonium bromide (CTAB), ascorbic acid (AA), sodium thiosulfate (Na2S2O3), Tween-20, ochratoxin A (OTA), T-2 toxin, fumonisin B1 (FB1) and AFB1 were purchased from Sigma Aldrich (Taufkirchen, Germany). Disodium hydrogen phosphate (Na2HPO4) and sodium dihydrogen phosphate (NaH2PO4) were purchased from Fluka (Buchs, Switzerland). Polyethylene glycol 8000 (PEG-8000) was obtained from Carl Roth (Karlsruhe, Germany). Phosphate buffer solution (PBS) was prepared by using 0.2 M NaH2PO4 and 0.2 M Na2HPO4 and then diluted to the corresponding concentration. The mouse monoclonal anti-aflatoxin antibody 1F2 was from our group.33 Ultrapure water was produced using reverse osmosis with UV treatment (Milli-RO 5 Plus, Milli-Q185 Plus, Millipore, Eschborn, Germany).Apparatus
UV-vis absorption spectra were measured on a Specord 250 Plus UV-vis spectrophotometer (Analytik Jena, Jena, Germany). RLS spectra were measured on a RF-5301 PC spectrofluorometer (Shimadzu, Tokyo, Japan) by simultaneously scanning the excitation and emission monochromators from 300 to 800 nm with Δλ = 0 nm and sensitivity set to low.Preparation of Ab-AuNPs and AFB1-BSA-Fe3O4 magnetic beads
Ab-AuNPs and MBs were prepared and characterized according to the published method.34 The AuNPs with size of 35 nm were used. The Ab-AuNPs were dispersed in 5 mM PBS (pH 7.4) containing 0.1% PEG-8000. The concentration of AuNPs and MBs was estimated to be 0.40 nM and 4 mg mL−1, respectively.Preparation of gold growth solution
General procedure: 25 μL of 0.1 M HAuCl4 was added to 10 mL of 100 mM CTAB in water. The mixture was heated in a water bath and mixed until all the precipitates were dissolved. Then 100 μL of 100 mM AA solution was added. The solution changed immediately from clear orange to colorless. The growth solution was ready for use after cooling down to room temperature.To study the influence of CTAB on gold enhancement, CTAB solution with different concentrations (0, 5, 10, 25, 50, 75, 100 mM) was used to prepare the gold growth solution. To investigate the effect of AA, solutions were tested with different final concentrations (0.5, 0.55, 0.6, 0.75, 1, 2, 5, 10 mM). Volumes of 10, 25 and 40 μL of HAuCl4 were used to evaluate the influence of gold concentration.
Growth of AuNPs in aqueous phase
The immunogold nanoparticles (∼0.20 nM Ab-AuNPs in 25 mM PBS, pH 7.4, 0.05% PEG-8000) were used as gold seeds for the enlargement. Generally, 50 μL of gold seeds were added to 1.0 mL of the growth solution. The absorption spectra were measured after 30 min. The kinetics of the AuNP growth was quantitatively monitored by UV-vis spectroscopy and RLS. To study the influence of immunogold concentration, different amounts of gold seeds (5–50 μL) were added to 1.0 mL growth solution for gold enhancement.Analysis of maize samples
Pulverized maize samples were purchased from supermarket in Munich. The maize samples (1 g) were spiked with AFB1 at different concentrations (0, 4, 8, 20 and 40 μg kg−1). The spiked samples were kept at room temperature under dark condition for 3 h to evaporate methanol used to prepare AFB1 standards and then extracted with 4 mL of methanol–water (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, v/v) by vortex mixing for 2 min and then centrifugated at 1920g for 15 min. The supernatant was then 20-fold diluted with PBS (50 mM, pH = 7.4) for quantitative analysis.
20, v/v) by vortex mixing for 2 min and then centrifugated at 1920g for 15 min. The supernatant was then 20-fold diluted with PBS (50 mM, pH = 7.4) for quantitative analysis.
Assay procedure
The AFB1 standard solutions with different concentrations were prepared in 50 mM PBS (pH 7.4). A volume of 50 μL of AFB1 standards/samples was injected into a 0.5 mL Eppendorf tube, then 50 μL of Ab-AuNPs and 15 μL of MBs suspension (∼4 mg mL−1) were added successively. The mixture was well mixed and incubated under shaking for 30 min. After removing the formed immune complexes (i.e. anti-AFB1-AuNP-AFB1-BSA-MBs) by magnetic separation, 100 μL of supernatant solution containing unbound AuNPs was transferred into 1.0 mL gold growth solution and the mixture was incubated at room temperature for 20 min. Then 100 μL of 10 mM Na2S2O3 was added to stop the reaction and the absorbance of the enlarged AuNPs was measured on UV-vis spectrophotometer. The absorbance at 565 nm (533 nm for AuNPs without gold staining) was recorded and final absorbance was calculated by subtracting the absorbance of the corresponding blank samples. Error bars were standard deviations across at least three repetitive assays.Results and discussion
The principle of the immunoassay is illustrated in Fig. 1. Bio-functionalized MBs and free AFB1 molecules competitively bind to AuNP-labeled antibodies. After magnetic separation, the supernatant containing unbound AuNPs was directly submitted for gold enlargement. The final absorbance depends on the amount of immunogold nanoparticles added, which is directly proportional to the concentration of AFB1 in the sample.Proposed mechanism of gold enlargement
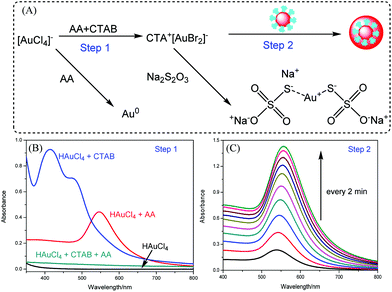
The proposed mechanism of the homogeneous growth of AuNPs is illustrated in Fig. 2A. The immunogold nanoparticles act as self-catalysts while the CTAB serves as a surfactant to stabilize the enlarged nanoparticles. Ascorbic acid was employed as the reducing agent for the gold enlargement.In the first stage, the Au3+ ions were rapidly reduced to Au+ ions by AA. The Au+ ions can be stabilized by CTAB. As shown in Fig. 2B, chloroauric acid solution had very low absorption between 350 nm and 800 nm. In the presence of CTAB, a significant absorption peak appeared at 416 nm with a shoulder around 470 nm. This might be ascribed to ligand exchange between HAuCl4 and CTAB. Au(III)Br4-CTA complex was formed, which takes on a clear orange color.35,36 After the addition of AA, the Au(III)Br4-CTA complex was rapidly reduced to Au(I)Br2-CTA, which was colorless. In the absence of CTAB, the Au3+ ions were directly reduced to Au0. An obvious absorption peak was observed at 550 nm, indicating the formation of small gold colloids. In the second stage, the Ab-AuNPs served as self-catalysts by receiving and transferring the electrons to Au+ species.25 These Au+ ions were then reduced to gold atoms which deposited onto the surfaces of Ab-AuNPs, resulting in the size growth of the AuNPs, which can be easily monitored by UV-vis spectroscopy, as indicated in Fig. 2C. The absorbance increased dramatically with increasing deposition time, while the absorption maximum was gradually red-shifted, which can be attributed to the increase of particle size and variations in the refractive index as well as partial aggregation of the AuNPs during enlargement.
Controlled growth of immunogold in aqueous solution
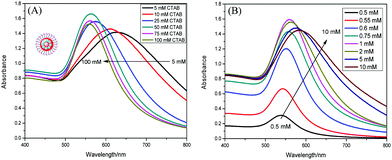
In order to optimize the gold enlargement, several experimental parameters were investigated in detail, including the concentrations of CTAB, AA and HAuCl4.Typically, the CTAB used in this study not only stabilizes the reduced Au+ ions but also is crucial for preventing the aggregation of enlarged AuNPs, which is very significant for assays based on optophysical properties of the nanostructures (e.g. absorption, light scattering). As shown in Fig. 3A, with the decrease of CTAB concentration, the absorption spectrum became broader and the maximum absorption was red-shifted. This might be attributed to the enlarged AuNPs aggregated at low concentration of CTAB due to high surface energy. Thus, a high concentration of CTAB, 100 mM, was used in further experiments. The AA reduced Au3+ to Au+, and the Au+ species were further reduced to gold atoms in the presence of immunogold. A certain amount of AA was required to reduce all the gold ions. As shown in Fig. 3B, the absorbance increased rapidly when the AA concentration rose from 0.5 to 1 mM, and then decreased slightly in the range of 2–10 mM. The maximum absorbance occurred at 1 mM AA. Therefore, 1 mM AA was used for the following studies.
 | ||
| Fig. 3 Effect of (A) CTAB concentration (0.25 mM HAuCl4 and 1 mM AA) and (B) AA concentration (100 mM CTAB and 0.25 mM HAuCl4) on the absorbance of the enlarged AuNPs. | ||
The influence of the concentration of HAuCl4 was also investigated as indicated in Fig. 4A. The absorbance at 550 nm was monitored by UV-vis spectroscopy. It is clearly seen that the gold enhancement was faster at higher concentration of HAuCl4. But at 0.4 mM HAuCl4, the absorbance decreased after 15 min. This might be caused by the enlarged AuNPs which are too big at high concentration of chloroauric acid and therefore, some particles precipitated, resulting in a decrease in the absorbance. Hence, 0.25 mM of chloroauric acid was selected for the following experiments.
The gold enlargement is time-dependent and normally the process will be catalyzed continuously until all the gold ions are exhausted. Thus, a long reaction time is required. Recently, we found that Na2S2O3 can efficiently stop the reaction. As shown in Fig. 4B, after the addition of Na2S2O3, the absorbance did not increase anymore and kept almost constant. This is because stable gold–sodium thiosulfate complex was formed as shown in Fig. 2A, which could not be reduced by AA and is well soluble in water. Thus, Na2S2O3 solution was used as stop solution for gold enlargement.
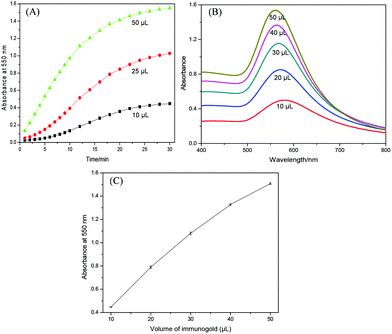
The homogeneous growth of immunogold was then conducted under optimized conditions. Fig. 5A shows the kinetics behavior of particle growth using different amounts of gold seeds. Various amounts of immunogold were added to 1.0 mL of growth solution and the absorbance was monitored. The absorption spectra after 30 min were shown in Fig. 5B. It can be clearly seen that the rate of gold enlargement and final absorption intensity are highly dependent on and proportional to the initial concentration of immunogold (Fig. 5C), which provides a quantitative basis for signal amplification.
Monitoring the gold enlargement by RLS spectra
The RLS spectra of enlarging AuNPs were also investigated. RLS occurs when the wavelength of incident light is close to that of the absorption band of metal nanoparticles.37 AuNPs exhibit characteristic RLS peak at about 550 nm. The RLS intensity can be greatly enhanced with increasing particle size. As shown in Fig. 6A, a typical RLS peak was observed around 555 nm. With the increase of deposition time, the RLS intensity increased dramatically. Different from the absorption band, the wavelength of RLS peak kept almost the same. Fig. 6B shows the kinetic plots of RLS intensity at 555 nm versus time. The RLS intensity increased faster at higher concentration of immunogold. Similar to the absorption behavior, the RLS intensity did not increase when the gold enlargement was stopped by the addition of Na2S2O3 (Fig. 6C). Thus, RLS technique could be utilized as another tool for monitoring the gold enhancement.Amplified optical detection of AFB1 through controlled growth of immunogold
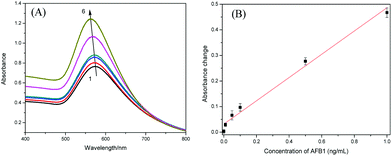
The AFB1 standards were quantitatively analyzed following the procedures described in the Experimental section. Fig. 7A shows the absorption spectra of supernatants after gold enhancement. With the increase of AFB1 concentration, the absorption intensity increased correspondingly, and the maximum absorption was slightly blue-shifted, which is ascribed to smaller enlarged AuNPs at higher concentration of immunogold. As seen in Fig. 7B, a linear dependence between absorbance change at 565 nm and AFB1 level could be achieved in the dynamic range from 0.01 to 1 ng mL−1 (ppb). The regression equation could be fitted to y = 0.4543 × C[AFB1] + 0.3182 (ng mL−1, R2 = 0.988, n = 6). The limit of detection (LOD) was estimated to be 0.007 ng mL−1 (7 ppt) based on three times of signal-to-noise ratio.The detectable concentration range of the developed assay is comparable with that obtained by an ELISA38 or an electrochemical immunosensor,39 which used the same anti-AFB1 antibody. In addition, the sensitivity of proposed assay format is also high. Fig. 8 shows the comparison of AFB1 detection without and with gold enlargement. After gold staining, the signal response increased distinctly. Take 1 ng mL−1 AFB1 for example, the absorbance change is about 3.5 times of that without gold staining, which indicates that the sensitivity was indeed improved after gold enhancement. Moreover, the method is relatively fast, with a total assay time of ∼1 h.
 | ||
| Fig. 8 Response curves of the developed immunoassay toward AFB1 standards: (a) without and (b) with gold enlargement (n = 3). | ||
Further, the specificity of the developed immunoassay was evaluated using common mycotoxins as competitors, including FB1, T-2 toxin, OTA, and their mixture with AFB1. As shown in Fig. 9, a significant change in absorbance was only observed in the presence of AFB1, which demonstrated that the assay has a good selectivity toward AFB1 detection.
 | ||
| Fig. 9 Specificity of the developed assay towards AFB1 (1 ng mL−1), FB1 (20 ng mL−1), T-2 toxin (20 ng mL−1), OTA (20 ng mL−1) and their mixture with AFB1 (n = 3). | ||
The feasibility of applying the established immunoassay for complex matrices was evaluated by the analysis of spiked maize samples. The results are summarized in Table 1. Satisfactory recoveries were obtained in the range of 90.7% to 115.1% with acceptable relative standard deviation (RSD). This analysis demonstrated that the established method could be used for quantitative monitoring of AFB1 in real samples.
| In sample (μg kg−1) | After dilution (ng L−1) | Detected value (ng L−1) | Recovery (%) |
|---|---|---|---|
| 4 | 50 | 51.5 | 103.0 ± 8.2 |
| 8 | 100 | 115.1 | 115.1 ± 13.2 |
| 20 | 250 | 255.6 | 102.2 ± 6.9 |
| 40 | 500 | 453.3 | 90.7 ± 2.1 |
The proposed method is simple, specific and highly sensitive. Compared with highly sensitive DNA amplification such as hybridization chain reaction (HCR)40 and loop mediated isothermal amplification (LAMP),41 the design and operation of the gold staining amplification method are much easier. Furthermore, the established method does not require expensive or challenging equipments. This strategy is very attractive for mycotoxin determination because (1) the preparation and the bio-functionalization of the nanoparticles is simple and generally applicable; (2) taking advantages of the amplification effect of catalytic deposition of gold together with the extremely high molar extinction coefficient of enlarged AuNPs, the gold enlargement-based colorimetric approach shows high sensitivity; (3) the use of magnetic particles as solid carrier reduces the incubation time, and facilitates the rapid separation of immune complexes.
Conclusions
We have shown here the controlled growth of AuNPs using CTAB as stabilizing surfactant, AA as reducing agent, and Na2S2O3 as stop reagent. Surface plasmon resonance signature of the enlarged AuNPs and the kinetics of the gold enlargement were monitored by UV-vis spectroscopy and RLS technique. The absorbance as well as RLS intensity of the enlarged AuNPs is highly dependent on the initial concentration of gold seeds. The controlled homogenous gold enlargement was then utilized for signal amplification in competitive immunoassay for the first time. High sensitivity and satisfactory recoveries in spiked maize samples were achieved for the detection of AFB1. The total assay is simple, specific, sensitive, and could be easily transferred to the detection of other toxins and organic pollutants.Acknowledgements
The financial support of the China Scholarship Council is gratefully acknowledged.Notes and references
- E. C. Dreaden, A. M. Alkilany, X. Huang, C. J. Murphy and M. A. El-Sayed, Chem. Soc. Rev., 2012, 41, 2740–2779 RSC.
- K. Saha, S. S. Agasti, C. Kim, X. Li and V. M. Rotello, Chem. Rev., 2012, 112, 2739–2779 CrossRef CAS PubMed.
- H. Jans and Q. Huo, Chem. Soc. Rev., 2012, 41, 2849–2866 RSC.
- K. E. Sapsford, W. R. Algar, L. Berti, K. B. Gemmill, B. J. Casey, E. Oh, M. H. Stewart and I. L. Medintz, Chem. Rev., 2013, 113, 1904–2074 CrossRef CAS PubMed.
- L. Dykman and N. Khlebtsov, Chem. Soc. Rev., 2012, 41, 2256–2282 RSC.
- J. Sun, Y. Xianyu and X. Jiang, Chem. Soc. Rev., 2014, 43, 6239–6253 RSC.
- D. Vilela, M. C. González and A. Escarpa, Anal. Chim. Acta, 2012, 751, 24–43 CrossRef CAS PubMed.
- Y. Song, W. Wei and X. Qu, Adv. Mater., 2011, 23, 4215–4236 CrossRef CAS PubMed.
- X. Cao, Y. Ye and S. Liu, Anal. Biochem., 2011, 417, 1–16 CrossRef CAS PubMed.
- W. Lai, D. Tang, J. Zhuang, G. Chen and H.-H. Yang, Anal. Chem., 2014, 86, 5061–5068 CrossRef CAS PubMed.
- D. Tang, B. Liu, R. Niessner, P. Li and D. Knopp, Anal. Chem., 2013, 85, 10589–10596 CrossRef CAS PubMed.
- W.-H. Zhou, C.-L. Zhu, C.-H. Lu, X. Guo, F. Chen, H.-H. Yang and X. Wang, Chem. Commun., 2009, 6845–6847 RSC.
- A. Ambrosi, F. Airo and A. Merkoçi, Anal. Chem., 2009, 82, 1151–1156 CrossRef PubMed.
- L. Zhan, W. B. Wu, X. X. Yang and C. Z. Huang, New J. Chem., 2014, 38, 2935–2940 RSC.
- W. Li, J. Li, W. Qiang, J. Xu and D. Xu, Analyst, 2013, 138, 760–766 RSC.
- X. Que, D. Tang, B. Xia, M. Lu and D. Tang, Anal. Chim. Acta, 2014, 830, 42–48 CrossRef CAS PubMed.
- W. Li, W. Qiang, J. Li, H. Li, Y. Dong, Y. Zhao and D. Xu, Biosens. Bioelectron., 2014, 51, 219–224 CrossRef CAS PubMed.
- R. Liu, X. Liu, Y. Tang, L. Wu, X. Hou and Y. Lv, Anal. Chem., 2011, 83, 2330–2336 CrossRef CAS PubMed.
- R. Liu, Y. Zhang, S. Zhang, W. Qiu and Y. Gao, Appl. Spectrosc. Rev., 2014, 49, 121–138 CrossRef CAS.
- Z. Ma and S. F. Sui, Angew. Chem., Int. Ed., 2002, 41, 2176–2179 CrossRef CAS.
- D. Kim, W. L. Daniel and C. A. Mirkin, Anal. Chem., 2009, 81, 9183–9187 CrossRef CAS PubMed.
- A. Fan, C. Lau and J. Lu, Analyst, 2009, 134, 497–503 RSC.
- V. Pavlov, Y. Xiao, B. Shlyahovsky and I. Willner, J. Am. Chem. Soc., 2004, 126, 11768–11769 CrossRef CAS PubMed.
- A. Fan, S. Cai, Z. Cao, C. Lau and J. Lu, Analyst, 2010, 135, 1400–1405 RSC.
- Z. Zhan, C. Cao and S. J. Sim, Biosens. Bioelectron., 2010, 26, 511–516 CrossRef CAS PubMed.
- C. Cao, X. Li, J. Lee and S. J. Sim, Biosens. Bioelectron., 2009, 24, 1292–1297 CrossRef CAS PubMed.
- P. Li, Q. Zhang, D. Zhang, D. Guan, X. Ding, X. Liu, S. Fang, X. Wang and W. Zhang, Aflatoxins: Detect., Meas. Control, 2011, 11, 183–208 Search PubMed.
- W. S. Khayoon, B. Saad, C. B. Yan, N. H. Hashim, A. S. M. Ali, M. I. Salleh and B. Salleh, Food Chem., 2010, 118, 882–886 CrossRef CAS PubMed.
- A. Bacaloni, C. Cavaliere, F. Cucci, P. Foglia, R. Samperi and A. Laganà, J. Chromatogr. A, 2008, 1179, 182–189 CrossRef CAS PubMed.
- B. B. Dzantiev, N. A. Byzova, A. E. Urusov and A. V. Zherdev, TrAC, Trends Anal. Chem., 2014, 55, 81–93 CrossRef CAS PubMed.
- D. Tang, Y. Lin, Q. Zhou, Y. Lin, P. Li, R. Niessner and D. Knopp, Anal. Chem., 2014, 86, 11451–11458 CrossRef CAS PubMed.
- S. Oswald, X. Karsunke, R. Dietrich, E. Märtlbauer, R. Niessner and D. Knopp, Anal. Bioanal. Chem., 2013, 405, 6405–6415 CrossRef CAS PubMed.
- C. Cervino, E. Weber, D. Knopp and R. Niessner, J. Immunol. Methods, 2008, 329, 184–193 CrossRef CAS PubMed.
- X. Wang, R. Niessner and D. Knopp, Sensors, 2014, 14, 21535–21548 CrossRef PubMed.
- Z. Khan, T. Singh, J. I. Hussain and A. A. Hashmi, Colloids Surf., B, 2013, 104, 11–17 CrossRef CAS PubMed.
- S. A. AL-Thabaiti, J. I. Hussain, A. A. Hashmi and Z. Khan, Can. Chem. Trans., 2013, 1, 238–252 CAS.
- W. B. Wu, L. Zhan, J. Wang and C. Z. Huang, Anal. Methods, 2014, 6, 3779–3783 RSC.
- D. Li, Y. Ying, J. Wu, R. Niessner and D. Knopp, Microchim. Acta, 2013, 180, 711–717 CrossRef CAS.
- D. Tang, Z. Zhong, R. Niessner and D. Knopp, Analyst, 2009, 134, 1554–1560 RSC.
- B. Zhang, B. Liu, D. Tang, R. Niessner, G. Chen and D. Knopp, Anal. Chem., 2012, 84, 5392–5399 CrossRef CAS PubMed.
- M. Dou, D. C. Dominguez, X. Li, J. Sanchez and G. Scott, Anal. Chem., 2014, 86, 7978–7986 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |