Presence of electrolyte promotes wetting and hydrophobic gating in nanopores with residual surface charges†
Abstract
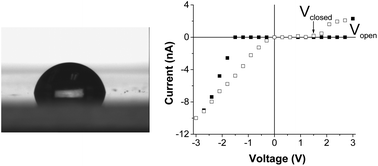
Hydrophobic nanopores provide a model system to study hydrophobic interactions at the nanoscale. Such nanopores could also function as a valve since they halt the transport of water and all dissolved species. It has recently been found that a hydrophobic pore can become wetted i.e. filled with condensed water or an aqueous solution of salt when a sufficiently high electric field is applied across the membrane. The wetting process is reversible thus when the voltage is lowered or switched off, the pore comes back to a closed state due to water evaporation in the pore. In this manuscript we present experimental studies on how the switching between conducting and non-conducting states can be regulated by the electrolyte concentration. Transport properties of single nanopores modified with alkyl chains of different lengths were recorded in salt concentrations between 10 mM and 1 M KCl. Nanopores modified with propyl chains exhibited gating in 10 mM KCl and were open for ionic transport for all voltages at higher salt concentrations. Nanopores modified with decyl chains did not conduct current in 10 mM and exhibited repeatable hydrophobic gating in 100 mM and 1 M KCl. The results are explained in the context of Maxwell stress in confined geometry with local surface charges, which change the shape of the water–vapor interface and promote wetting.
- This article is part of the themed collection: From nanopores to nanochannels

 Please wait while we load your content...
Please wait while we load your content...