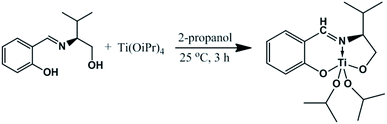Solvent-free ring opening polymerization of ε-caprolactone and electrical properties of polycaprolactone blended BiFeO3 nanocomposites
Somasundaram Saravanamoorthya,
Muniyandi Muneeswaranb,
NambiVenkatesan Giridharanb and
Sivan Velmathi*a
aOrganic and Polymer Synthesis Laboratory, Department of Chemistry, National Institute of Technology, Tiruchirappalli 620 015, India. E-mail: velmathis@nitt.edu; Fax: +91 431 2500133; Tel: +91 431 2503640
bAdvanced Functional Materials Laboratory, Department of Physics, National Institute of Technology, Tiruchirappalli 620 015, India
First published on 21st April 2015
Abstract
A novel two-phase polymer nanocomposite comprising polycaprolactone (PCL) and nanocrystalline multiferroic BiFeO3 (BFO NPs) has been fabricated by a simple casting technique. We have developed a new Schiff base ligand–Ti(IV) catalyst based on tridentate ONO chiral amino alcohol, which has been derived from salicylaldehyde. This catalyst plays a vital role in the ring opening polymerization (ROP) of cyclic esters such as ε-caprolactone (ε-CL). Nanocomposites consisting of multiferroic BFO NPs and insulating PCL have been prepared. It was found that the prepared nanocomposites PCL/BFO/1 wt% and PCL/BFO/3 wt% exhibit ferroelectric properties and reduced leakage of current density. Dielectric data were analyzed using the complex permittivity and complex electric modulus for the nanocomposites at room temperature. An improved AC conductivity for the PCL/BFO nanocomposites was observed compared to the pure PCL and BFO samples.
1. Introduction
Recently, much effort has been devoted to the synthesis of polycaprolactone (PCL), not only because of its immense applications in the field of medicine but also for its versatile properties in agriculture1 and materials science.2 Synthetic polymers have become an indispensable part of daily-life for human beings and the biodegradable class of polymers offer immense value in therapeutics.3 Among the commercially viable polymers, PCL is an important polymer due to its mechanical properties, biodegradability and its miscibility with a large range of other polymers.4 We can list the promising applications of PCL in medicine and tissue engineering as it has been used in drug delivery media for the controlled release of drugs and scaffolds. Controlled release plays an important role in the delivery of antibodies and genes,5 and also in the development of efficient catalytic systems. As a result of these applications, the preparation of PCL has drawn much attention in both academia and industry. The prime method to synthesize PCL is by the ring opening polymerization (ROP) of ε-caprolactone (ε-CL) with metal alkoxides as initiators. ROP can be classified based on the usage of solvent into (i) solution polymerization and (ii) solvent-free (bulk) polymerization. Among these two, bulk polymerization is preferred because it offers the following advantages: (i) no requirement for solvent, (ii) less vulnerability to impurity levels and unwanted side reactions and (iii) large-scale industrial production.6 Most of the solvent-free bulk methods show improved selectivity, reduced reaction time, and simplified separation and purification of products.7 It is remarkable to note that the polyesters prepared using this method have high commercial value as they exhibit good mechanical strength, high crystallinity, biodegradability and adaptable functionalization possibilities. Well-defined metal catalysts or initiators have been reported for ROP, including aluminium,8 titanium,9 tin,10 zinc11 and magnesium12 complexes supported by various ligands.These catalysts give polymers with high molecular weights in high yields. Among the above mentioned complexes, titanium alkoxide based initiator systems seem to be very active and suitable for preparing PCL due to their high Lewis acid nature. However, when metal alkoxides are used as initiators for the ROP of lactones and lactides, they lead to undesirable back-biting and transesterification reactions. This results in macrocycles, catalyst inhomogeneity, and broad or multimodal polymer molecular weight distributions. F. Peruch et al.,13 synthesized Ti(IV) complexes with O,N,O-tridentate aminodiol ligands and these Ti(IV) complexes were identified as efficient catalysts for the controlled ROP of L and D,L-lactide. Inspired by these results, we postulated that the Ti(IV) complex of (S,E)-2-(((1-hydroxy-3-methylbutan-2-yl)imino)methyl)phenol (LVTi) could demonstrate high catalytic activity in a ROP reaction. Catalysis with Schiff-base metal complexes in various chemical reactions, both in homogeneous and heterogeneous media, is widely known.14 However, the efforts that have been dedicated to their use in ROP reactions are relatively limited.15 Due to their Lewis acidity, Ti(IV) complexes have been widely used as a catalyst in ROP reactions. Additionally, the transition metal titanium is fairly cheap compared to noble metals like Ag, Ru, Pt, Pd and Au.
Recently, polymer based ferroelectric (FE) materials, such as flexible higher particulate ceramic/polymer composites, have been identified as crucial components for use in advanced electronic devices,16,17 for instance, as memory and gate dielectrics in integrated circuits,18,19 and as miniature capacitors for telecommunications.20–22 Only a few reports have been focused on the multiferroic properties of polymer/alloy-based and metal/ceramic based composites. However, due to the large current leakage and ease of oxidation, these alloy-based magnetostrictive materials have some limitations when being used as a nanocomposite. Moreover, a nanoscale structure has a major effect on the dielectric and ferroelectric properties of the composites. This is an important issue for other nanocomposites and so their shapes and sizes need to be easily modified by conventional polymeric processing. This helps to increase the electrostriction of the composites. In addition, many FE ceramics contain lead based oxides, which are not environmentally friendly. Another class of recently discovered interesting ceramic materials is the multiferroic materials (MFMs), which have both magnetic and FE properties. These materials have drawn much attention as prospective candidates for their potential applications in emerging fields like information storage, spintronics, and sensor devices.23,24 Very recently, multiferroic behavior has been observed in polymer composites consisting of MFMs.25,26 BFO is one vastly studied MFM system with a high FE Curie temperature (TC ∼ 830 °C).
Some metal catalysts used to prepare polymers have failed to find industrial application due to their high binding affinity to the polymer, which makes removal of the catalyst from the polymer difficult. Our objective is to investigate the catalytic activity of LVTi for the ROP of ε-CL under solvent free conditions. The amount of catalyst used in the present study is very low compared to the results reported in the literature. In this work, we have reported a simple method for the fabrication of PCL/BFO nanocomposites, a new kind of lead (Pb) free and low dielectric loss material. PCL has been selected as the polymer host for its well-known commercial applications. PCL is a semicrystalline polymer, which combines 50% crystallinity with good mechanical properties, chemical resistance, electrical resistance and processability. Thus, we studied the electrical properties of the BiFeO3 nanocomposites using the PCL synthesized by our catalyst, as commercially available PCL is of high cost. In the present study, the catalyst can be removed and recycled, thus metal contamination in the synthesised PCL is less, which is one of the advantages of our work. Our work in this article is mainly focused on enhancement of the ferroelectric properties, reduction of the leakage of current density and improvement of AC electrical conductivity by using BFO NPs as a suitable multiferroic filler.
2. Experimental
2.1 Materials
L-Valine (Merck), iodine (Merck), sodium borohydride (Merck), hexane (Merck), salicylaldehyde (Merck), potassium hydroxide (Rankem), ethanol (Merck), methanol (Rankem), chloroform (Merck), tetrahydrofuran (Rankem), titanium isopropoxide (Sigma Aldrich), 2-propanol (Merck), ε-caprolactone (Sigma Aldrich), bismuth nitrate (Merck), iron(III) nitrate nonahydrate (Merck) and ammonium hydroxide (Merck) were used as received. Tetrahydrofuran was used only after distillation with sodium metal and benzophenone.2.2 Characterization
The 1H and 13C NMR spectra were recorded on a Bruker 400 MHz NMR spectrometer in an appropriate solvent (DMSO-d6 or CDCl3) using tetramethylsilane (TMS) as an internal standard. FT-IR spectra were recorded by a Thermo IS5 FT-IR spectrophotometer and wide-angle X-ray diffraction (WAXD) analysis was carried out using a Rotaflex RTP300 X-ray diffractometer (Rigaku Co., Tokyo, Japan). A Shimadzu UV-2600 UV-vis spectrophotometer was used to record UV-visible spectra using a quartz cell with a 1 cm path length. The mass spectra were recorded using an ESI technique on a SYNAPT G2 mass spectrometer. For DSC (differential scanning calorimeter DSC Q10 V9.4 Build 275) analysis, approximately 5–10 mg of sample was weighed and spread uniformly in a hermetic aluminum pan to ensure proper thermal contact. Microstructure and morphology studies were performed using a field emission scanning electron microscope (Nova NanoSEM NPE 206 high resolution SEM). Dielectric and AC conductivity studies were performed with a LCR meter (HIOKI 3532-50, Japan). Leakage measurements were carried out with a Keithley 6517, and ferroelectric studies were performed with a P–E loop tracer (Precision II, Multiferroic Tester, Radiant Technologies, USA). The number and weight average molecular weight (Mn and Mw) as well as the polydispersity (Mw/Mn) index (PDI) of the polymer samples were measured by gel permeation chromatography (GPC) using a Waters HPLC system equipped with a model 515 EF binary pump, model 2707 auto injector, model 2414 refractive index detector (RI), and Waters styragel columns (HR 3 and HR 4). THF (HPLC grade) was used as an eluent at a flow rate of 1.0 mL min−1. The sample concentration and injection volume were 0.25% (w/v) and 50 μL respectively. Empower Waters software was used to calculate molecular weights based on a calibration curve generated by the narrow molecular weight distribution of the polystyrene standards.2.3 Synthesis of the ligand
(S)-2-Amino-3-methyl-1,1-diphenylbutan-1-ol (LV) was synthesized as per the previously reported procedure.27,28 The prepared chiral Schiff base was characterized by 1H NMR, 13C NMR and FT-IR.LV: melting point: 99–102 °C, [α]25D = −24.1° (c = 1, CH3OH). FT-IR (cm−1) 3580, 2962, 1628, 1278, 1075. 1H NMR (CDCl3, δ ppm): 0.88 (dd, 3J = 1.2 Hz, 6H, –(CH3)2), 1.87 (m, 3J = 6.4 Hz, 1H, –CH), 2.99 (m, 3J = 2.8 Hz, 1H, –CH), 3.69 (dd, 3J = 2.4 Hz, 1H, –CH), 3.76 (dd, 3J = 3.6 Hz, 1H, –CH), 5.04 (s, 1H, OH), 6.82 (t, 3J = 6.4 Hz, 1H, –CH), 6.88 (d, 3J = 8.4 Hz, 1H, –CH), 7.19 (t, 3J = 7.6 Hz, 1H, –CH), 7.25 (d, 3J = 7.2 Hz, 1H, –CH), 8.29 (s, 1H, –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 7.8 (s, 1H, –OH). 13C NMR (CDCl3, δ ppm): 17.85, 32.15, 62.1, 79.81, 117.3, 120.5, 123.7, 130.1, 133.6, 151.4, 161.53.
N), 7.8 (s, 1H, –OH). 13C NMR (CDCl3, δ ppm): 17.85, 32.15, 62.1, 79.81, 117.3, 120.5, 123.7, 130.1, 133.6, 151.4, 161.53.
2.4 Preparation of complex
Ti(OiPr)4 (0.85 g, 3 mmol) in 10 mL of isopropyl alcohol was cooled to 0 °C. Subsequently, the required LV (0.618 g, 3 mmol) in 10 mL of isopropyl alcohol was added drop wise to the reaction mixture with vigorous stirring. The mixture was then stirred at room temperature (25 °C) for 3 h to give a clear orange yellow solution (Scheme 1). The solvent was then removed under vacuum to yield the titanium isopropoxide complex as a crystalline orange yellow solid (0.98 g, 88% yield).Melting point: >320 °C, [α]25D = −125.25 (c = 1, CH3OH). Mass spectrum: molecular ion peak at 370 m/z. 1H NMR (DMSO-d6, δ ppm) 0.89 (m, –CH3), 1.01 (m, –CH3), 1.05 (m, –CH3), 1.72 (m, –CH3), 2.51 (s, J = 5.9 Hz, –CH), 3.58 (m, J = 1.93 Hz, –CH), 3.88 (d, J = 2.84 Hz, –CH), 4.1 (d, J = 4.09, –CH), 6.55 (m, J = 5.20, –CH), 7.22 (m, J = 3.63 Hz, –CH), 7.45 (t, J = 2.75 Hz, –CH), 7.68 (d, J = 1.19 Hz, –CH), 8.81 (s, J = 3.19 Hz, –CH). 13C NMR (400 MHz, DMSO-d6) 18.25, 19.30, 30.78, 32.07, 59.58, 71.34, 72.59, 117.70, 118.18, 121.76, 135.35, 135.48, 164.23, 165.02. FT-IR (KBr, cm−1) 3415, 2958, 1618, 1275, 1075, 1025. Elemental anal. calc. for C18H29NO4Ti: C, 58.23; H, 7.87; N, 3.77; O, 17.24; Ti, 12.89. Found: C, 57.11; H, 7.28; N, 3.87.
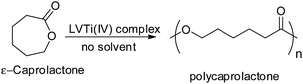
2.5 ROP reaction of ε-CL
The polymerization reactions were carried out in an Ace pressure tube in the presence of a pure nitrogen atmosphere with homogeneous heating. 0.005 g (0.014 mmol) of catalyst (LVTi) was added to 0.97 mL (1.0 g, 8.8 mmol) of ε-CL and the obtained mixture was heated at 125 °C under a N2 atmosphere for 24 h (Scheme 2). Succeeding the completion of the reaction, the product was later dissolved in 10 mL of chloroform and centrifuged. The obtained solution was poured into an excess of methanol. The white precipitate (PCL) obtained was separated via filtration, washed several times using methanol and dried for 8–10 h under vacuum. Yield 0.96 g (96%), Mw = 42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. IR (KBr disc, cm−1): 1727 (–C
000. IR (KBr disc, cm−1): 1727 (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2864 (–CH2), 2942 (–CH2), 3433 (–OH). 1H NMR (CDCl3, δ ppm): 2.30 (t, 2H, –CH2), 1.65 (m, 2Hs, –CH2), 1.40 (m, 2H, –CH2), 4.04 (t, 2H, –CH2).
O), 2864 (–CH2), 2942 (–CH2), 3433 (–OH). 1H NMR (CDCl3, δ ppm): 2.30 (t, 2H, –CH2), 1.65 (m, 2Hs, –CH2), 1.40 (m, 2H, –CH2), 4.04 (t, 2H, –CH2).
2.6 Synthesis of BiFeO3 NPs
The procedure followed for the synthesis of the BFO NPs was similar to one found in the literature.29 The synthesis procedure is as follows: a combination of Bi(NO3)3·5H2O and Fe(NO3)3·9H2O was dissolved in 200 mL of double distilled water and stirred well for 20 minutes to obtain a clear solution. The reaction product was obtained by the synchronous dropwise addition of ammonia solution and distilled water. The resulting precipitate was maintained at room temperature for about 24 hours, then washed several times with double distilled water to remove the unreacted products and filtered. Afterwards the final product was dried in a hot air oven at 100 °C for about 5 hours. Lastly, the final sintering was carried out at 600 °C for 2 hours.2.7 Synthesis of PCL/BiFeO3 nanocomposite materials
PCL/BFO NP nanocomposites were prepared by a solution casting method with different weight percentages of BFO NPs (1 and 3 wt%). PCL and BFO NPs were weighed, dissolved separately in 30 mL chloroform, and later stirred for 30 minutes at room temperature. These solutions were then mixed and stirred for 1 hour to obtain a homogeneous solution. Additionally, the solution was cast in a glass Petri dish and dried at the solvent atmosphere to obtain a solid sample. The obtained nanocomposites were characterised by various techniques such as FE-SEM, XRD and DSC.3. Results and discussion
3.1 Characterization of the catalyst
The chiral Schiff base ligand was synthesized by reacting L-valinol with salicylaldehyde, following a literature procedure.25 The titanium complex LVTi was prepared by reacting one equivalent of the metal precursor Ti(OiPr)4 with the same equivalent of the corresponding ligand (LV) (Scheme 1). UV spectral studies provided a useful insight into complex formation. The recorded spectra for LV and LVTi are shown in Fig. 1. From Fig. 1, it can be seen that the pure LV exhibits two intensive bands at 258 and 316 nm which confirms the π–π* and n–π* transitions respectively. However, LVTi shows an intense band with an observable blue shift at 256 nm and a new broad band appears at around 362 nm. These observations are attributed to metal to ligand charge transfer (MLCT) transitions. The consequence of coordination to the metal confirms the formation of the LVTi complex which causes the shift towards shorter wavelength.In order to confirm the structure of the synthesized complex, 1H & 13C NMR spectra were also measured. The obtained results signify co-ordination of Ti metal to hydroxyl-OH groups and an azomethine (–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–) group. All the protons were shifted downfield after complexation. Interestingly, it has been observed that the hydroxyl-OH protons disappear during complex formation. Moreover the azomethine proton of LV (8.29 ppm) suffers a significant downfield shift (to 8.9 ppm) upon coordination to the Ti(IV) metal ion. In fact the azomethine proton was deshielded, which was responsible for the observed downfield shift. Furthermore, the peaks due to the methyl and methane protons of the isopropyl groups (Ti–O–CH(CH3)2) were detected at 0.9–1.2 ppm (doublets) and 4.7 ppm (septets) respectively. The complex formation was further confirmed by 13C NMR spectroscopy as follows.
N–) group. All the protons were shifted downfield after complexation. Interestingly, it has been observed that the hydroxyl-OH protons disappear during complex formation. Moreover the azomethine proton of LV (8.29 ppm) suffers a significant downfield shift (to 8.9 ppm) upon coordination to the Ti(IV) metal ion. In fact the azomethine proton was deshielded, which was responsible for the observed downfield shift. Furthermore, the peaks due to the methyl and methane protons of the isopropyl groups (Ti–O–CH(CH3)2) were detected at 0.9–1.2 ppm (doublets) and 4.7 ppm (septets) respectively. The complex formation was further confirmed by 13C NMR spectroscopy as follows.
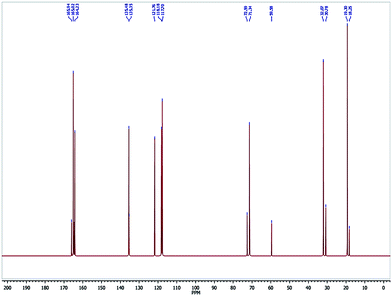
In the 13C NMR spectrum of LVTi, two aliphatic carbon peaks for the newly formed isopropyl groups are observed at 30 and 59 ppm (Fig. 2). Five aromatic carbon peaks are shown in the range 115–135 ppm and the remaining peak at δ 164 ppm is attributed to the oxygen attached to the quaternary carbon (C–O), which is shifted into the downfield region more than the corresponding ligand (δ 151 ppm). This is in good agreement with complex formation. Finally the azomethine carbon is shifted from 161 ppm to 165 ppm. These statistics clearly reveal complex formation.
3.2 Optimization of the reaction conditions for the ROP reaction of ε-CL
The chiral LVTi Schiff base complex was used as a catalyst for the ROP of ε-CL under solvent free conditions. To optimize the reaction conditions, the polymerization reaction was carried out with different amounts of catalysts such as 5, 2.5, 1.5 and 1 mg. The temperatures were kept at 150 and 125 °C for various reaction times such as 6, 12 and 24 h. The obtained results for PCL synthesis under these different conditions are given in Table 1. The optimum values for reaction temperature, reaction time and catalyst amount were determined to be 150 °C, 24 h and 5 mg (Table 1, entry 2) for the LVTi catalyst. For comparison, the same polymerization reaction was carried out with pure LV (Table 1, entry 18), Ti(OiPr)4 (Table 1, entry 19) and no catalyst (Table 1, entry 1) and they showed an inferior catalytic performance as opposed to LVTi.| Entry | [M]/[C]a ratio | Temperature (°C) | Time (h) | Mnd × 103 (g mol−1) | Mwe × 103 (g mol−1) | Yieldf (%) | PDIg |
|---|---|---|---|---|---|---|---|
| a [M]/[C] ratio is the molar ratio of monomer to catalyst.b [M]/[C] ratio is the molar ratio of monomer to ligand (LV).c [M]/[C] ratio is the molar ratio of monomer to Ti(OiPr)4.d Mn is the relative number-average molecular weight.e Mw is the relative weight-average molecular weight.f Calculated on the basis of the polymer weight.g PDI = Mw/Mn. | |||||||
| 1 | — | 150 | 24 | — | — | — | — |
| 2 | 653![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
150 | 24 | 31.66 | 42.12 | 96 | 1.33 |
| 3 | 653![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
150 | 12 | 20.60 | 31.73 | 95 | 1.54 |
| 4 | 653![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
150 | 6 | 12.61 | 21.52 | 90 | 1.70 |
| 5 | 1308![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
150 | 24 | 27.38 | 36.00 | 95 | 1.31 |
| 6 | 1308![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
150 | 12 | 13.79 | 16.79 | 92 | 1.21 |
| 7 | 1308![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
150 | 6 | 7.81 | 12.33 | 90 | 1.57 |
| 8 | 2612![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
150 | 24 | 14.11 | 22.71 | 92 | 1.60 |
| 9 | 2612![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
150 | 12 | 7.08 | 11.32 | 90 | 1.59 |
| 10 | 2612![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
150 | 6 | 4.72 | 9.41 | 87 | 1.99 |
| 11 | 3265![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
150 | 24 | 9.40 | 15.15 | 93 | 1.61 |
| 12 | 3265![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
150 | 12 | 6.03 | 9.10 | 91 | 1.50 |
| 13 | 3265![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
150 | 6 | 3.97 | 4.82 | 86 | 1.21 |
| 14 | 653![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
125 | 24 | 22.96 | 34.55 | 94 | 1.50 |
| 15 | 1308![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
125 | 24 | 19.55 | 31.98 | 90 | 1.63 |
| 16 | 2612![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
125 | 24 | 9.20 | 13.07 | 91 | 1.42 |
| 17 | 3265![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
125 | 24 | 7.24 | 11.53 | 86 | 1.59 |
| 18 | 366![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1b 1b |
150 | 24 | 1.46 | 1.77 | 74 | 1.20 |
| 19 | 517![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1c 1c |
150 | 24 | 6.31 | 9.83 | 84 | 1.55 |
The 1H NMR spectrum of PCL (Fig. 3) showed a significant signal at δ 3.66 ppm (triplet) corresponding to the –CH2–OH end group in PCL. Two signals at 1.35 and 4.2 ppm were also observed for PCL due to the presence of the iso-propyl ester end group.30 13C NMR (CDCl3, δ, ppm): 34.12 (–CH2), 28.34 (–CH2), 24.57 (–CH2), 25.52 (–CH2), 64.16 (–CH2), 173.58 (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) showed the respective number of carbons at appropriate regions.
O) showed the respective number of carbons at appropriate regions.
 | ||
| Fig. 3 1H NMR spectrum of PCL, synthesized by a ROP method using the LVTi complex, and illustrating the end group analysis. | ||
3.3 Characterization of nanocomposites
DSC was measured in the temperature range between 30–250 °C for pure PCL and PCL blended with BFO samples. It was observed that the melting temperatures of PCL and the PCL/BFO/3 wt% nanocomposite were 55 °C and 58 °C respectively. The observed increase in the melting temperature (Tm) of PCL/BFO/3 wt% is attributed to the addition of BiFeO3 NPs, which assist in producing the crystallization grains and forming a more regular structure within PCL. Hence, the Tm of the nanocomposites is slightly elevated compared with that of the pure PCL matrix, and the melting point levels off with increasing PCL/BFO/3 wt% content. Moreover, it can also be considered that when the inorganic grains of BFO are added into the PCL system, they hinder the large movement of the PCL long chains and it becomes inconvenient for the chains to move freely to change their configuration.31 Both of these two factors and their synergistic effect result in the shift of the Tm of the PCL/BFO/3 wt% nanocomposite. The X-ray diffraction pattern of pure PCL, pure BFO NPs, and the PCL/BFO/1 wt% and PCL/BFO/3 wt% nanocomposites show their crystalline behaviour (Fig. 4). The PCL sample shows three crystalline peaks at 21.08°, 21.87° and 23.34°, corresponding to the (110), (111), and (200) planes.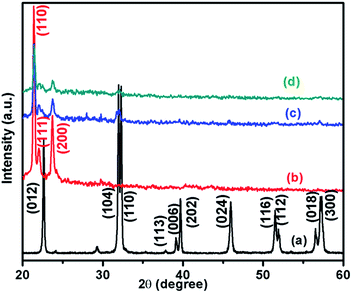 | ||
| Fig. 4 X-ray diffraction patterns of (a) BFO NPs, (b) PCL, (c) PCL/BFO/1 wt%, and (d) PCL/BFO/3 wt%. | ||
The XRD data demonstrate the highly ordered chain folding characteristics of the samples.30 BFO NPs exhibit a single-phase structure, and the diffraction peaks are indexed to be rhombohedral BiFeO3 with the space group R3c (JCPDS card no. 71-2494). XRD data were collected at a slow scan rate of 0.05° min−1. The diffractogram of the pure BFO NPs shows sharp peaks at 22.6°, 31.8°, 32.12°, 39.75°, 45.95°, 51.45° and 57.25° corresponding to the (012), (104), (110), (202), (204), (116) and (500) planes. This depicts their highly crystalline nature.29 As well, the XRD patterns of the PCL/BFO/1 wt% and PCL/BFO/3 wt% nanocomposites showed that the peaks were destabilized when compared with those of pure PCL and pure BFO NPs. This confirms the formation of the nanocomposites.
FE-SEM images show further evidence for formation of the nanocomposites. The FE-SEM microstructure and morphology of (a) PCL, (b) BFO NPs, (c) the PCL/BFO/1 wt% nanocomposite and (d) the PCL/BFO/3 wt% nanocomposite, on the same scale of 10 μm, are shown in Fig. 5. A cloud like appearance was observed for the PCL microstructure and (b) shows pure BFO NPs with an average size of 100 nm. NPs have been homogeneously and compactly dispersed in the PCL matrix to form a glans penis structure. As shown in Fig. 5(c and d), both PCL/BFO/1 & 3 wt% composites showed a more homogeneous, well stretched and single phase morphology. This demonstrates the good interaction of the components within the matrix.
3.4 Dielectric and electric modulus properties
Studies on dielectric properties are important to understand the nature and origin of dielectric losses, which, in turn, may be useful in the determination of structure and defects within solids. The real part of the dielectric constant is derived from the following relation,
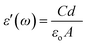 | (1) |
The frequency dependence of the dielectric constant ε′ and dielectric loss ε′′ at room temperature and at the frequency range of 100 Hz to 1 MHz were measured for the samples and shown in Fig. 6. It was observed that the prepared BFO NPs had higher dielectric constant values compared to the prepared PCL/BFO/1 wt% and PCL/BFO/3 wt%. This may be attributed to the space charge polarization near the grain boundary which depends on the high purity and perfection of the sample.
Also, the dielectric constant and dielectric loss values decreased gradually as the frequency increased from lower frequency and then become almost constant up to 1 MHz for all the sample values. These observations may be explained by the phenomenon of dielectric dispersion. Such a strong dispersion seems to be a common feature in ferroelectric materials concerned with ionic conductivity, which is referred to as low-frequency dielectric dispersion.32,33
The dielectric properties of materials can be expressed in various ways, with different representations. Although these alternative representations are equally valid, they may often provide new insight into the dielectric and electrical properties of materials. Therefore, many researchers prefer to describe the dielectric properties by using the electric modulus formalism.
The complex electric modulus has been derived from the complex permittivity,34 according to the relationship defined by Macedo et al.35 The complex electric modulus was calculated from the complex permittivity. The real (M′) and imaginary (M′′) parts of the electric modulus can be calculated from ε′ and ε′′ as follows:
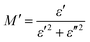 | (2) |
 | (3) |
The frequency dependences of the complex modulus spectra (M′ and M′′) of BFO, PCL, and the PCL/BFO/1 wt% and PCL/BFO/3 wt% nanocomposites are shown in Fig. 7(a) and (b). BFO has been characterized by very low values (almost zero) for M′ and M′′ in the frequency region of 1 KHz to 1 MHz.
The variation of M′ with frequency for PCL, and the PCL/BFO/1 wt% and PCL/BFO 3 wt% nanocomposites, results in the initial increase in magnitude of the electric modulus with increasing frequency, afterwards a fall in the electrical modulus is observed. This is attributed to the presence of conduction phenomena due to the short-range mobility of charge carriers.36 Variation of M′′ as a function of frequency for all the nanocomposites is shown in Fig. 7(b). The width of the M′′ peak was found to increase with an increase in frequency and its magnitude decreases with an increasing content of BFO NPs suggesting an enhancement in the capacitance of the nanocomposites. Two maximum peaks are observed, the first peak at lower frequency corresponds to the capacitance of grain boundaries and the second peak at higher frequency corresponds to grain capacitance.
3.5 Impedance spectra and AC conductivity
The complex impedance measurements of AC conductivity were done based on studies made on measurements of cell impedance. This is shown by plotting the relationship between Z (impedance), and Y (admittance) in a complex plane which is known as a Nyquist diagram.| Z = Z′ + Z′′ | (4) |
Z′ = |Z|![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ θ
| (5) |
Z′′ = |Z|![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ θ
| (6) |
In general, Z for both parts is frequency dependent. Impedance spectroscopy consists of the measurement of Z(ω) over a wide range of frequencies. The relation between the imaginary part (Z′′) and real part (Z′) is plotted in Fig. 8. It was observed that the magnitude of both Z′ and Z′′ decreased with an increase in frequency. This indicates the increase in AC conductivity with a rise to higher frequency.
 | ||
| Fig. 8 (a) Plot of the imaginary part and real part of impedance and (b) variation of AC conductivity with respect to frequency. | ||
When the impedance for the BFO NPs sample is very high, the impedance plot (inset graph Fig. 8) shows a straight line and not a semicircular arc curve over the measured frequency region. Only a single semicircle could be traced at room temperature for PCL and the PCL/BFO/1 wt% and PCL/BFO/3 wt% nanocomposites. As BFO NPs mixed with the polymer samples, the diameter for the impedance plots decreased. This demonstrates the decreased impedance of all the nanocomposites.
The AC conductivity (σ) was calculated from the following equation37
σ = εoε′′ω![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) tan tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ δ
| (7) |
The variation of AC conductivity with frequency for all the samples is shown in Fig. 8. The BFO NPs, PCL, and PCL/BFO/1 wt% and PCL/BFO/3 wt% nanocomposites show an increase in AC electrical conductivity with an increase in frequency. For all the nanocomposite samples, the AC conductivity values are the same at low frequencies and a further increase in conductivity was observed after 15 kHz, which is attributed to atomic and electronic polarizations.38,39 It is clear from these observations that the AC conductivity value of BFO NPs is found to be one order of magnitude less than those of the PCL, PCL/BFO/1 wt% and PCL/BFO/3 wt% samples.
3.6 The leakage current density
To investigate the leakage behaviour (Fig. 9(a)–(d)) of the prepared pure BFO NPs, pure PCL, and the PCL/BFO/1 wt% and PCL/BFO/3 wt% nanocomposites, the samples were subjected to an electric field of up to ±90 V cm−1 at room temperature. The results signified that current density increased with an increase in electric field for all of the compositions. The leakage current was then observed for both forward and reverse bias conditions, which was attributed to the bulk-limited conductions in BFO ceramics. The leakage current in BFO is usually attributed to the space charges (such as oxygen vacancies) induced mainly by Bi volatilization.40 At an applied electric field of 65 V cm−1, the leakage current density of PCL/BFO/3 wt% is about 1.3 × 10−7 A cm−2 which is approximately one order of magnitude less than that of PCL and BFO NPs. | ||
| Fig. 9 Electric field (E) dependence of leakage current density (J) of (a) BFO NPs, (b) PCL, (c) PCL/BFO/1 wt%, and (d) PCL/BFO/3 wt%. | ||
3.7 Ferroelectric properties
The electric field dependent polarization measurements for the prepared pure BFO NPs, pure PCL, PCL/BFO/1 wt% and PCL/BFO/3 wt% samples have been performed at two different frequencies and are shown in Fig. 10(a)–(d). The values of remnant polarization for the prepared pure BFO NPs, pure PCL, PCL/BFO/1 wt% and PCL/BFO/3 wt% samples at 500 Hz are 0.025, 0.011, 0.089 and 0.122 μC cm−2, respectively. An enhancement in remnant polarization occurs with the increasing amount of BFO NPs blended with the PCL samples. It has been found that the hysteresis loops of BFO ceramics become slimmer with the increasing frequency, which indicates that the remnant polarization and the coercive electric field decrease as frequency increases. At low frequency, there was electron displacement, ion displacement, turning-direction polarization and space charge polarization observed for BFO. But as the frequency increased, the space charge polarization could not keep up with the change of electric field so the remnant polarization and the coercive field tended to decrease.41 | ||
| Fig. 10 Polarization versus electric field (P versus E) hysteresis loops of (a) BFO NPs, (b) PCL, (c) PCL/BFO/1 wt%, and (d) PCL/BFO/3 wt% samples measured at different frequencies. | ||
4. Conclusions
In summary, we can assure that this is the first report on the study of BFO NPs blended with PCL along with a comparative study on the prepared pure BFO NPs, PCL, and the PCL/BFO/1 wt% and PCL/BFO/3 wt% nanocomposites. A chiral LVTi complex was successfully synthesized and structurally characterized. The characterized LVTi complex was used as an efficient catalyst for the ROP of ε-CL using a solvent free process. The prepared nanocomposites, PCL/BFO/1 wt% and PCL/BFO/3 wt%, have their own significant impact in terms of both ferroelectric and reduction of leakage current density properties. Moreover, the remnant polarization of the nanocomposites was higher compared with that of the pure BFO NPs. The AC conductivity of the BFO NPs was found to be one order of magnitude less than those of the PCL, PCL/BFO/1 wt% and PCL/BFO/3 wt% samples.Acknowledgements
The authors gratefully acknowledge funding support from the Council of Scientific & Industrial Research (CSIR) Sanction no. 01(2282)/08/EMR-II. DST (Sanction no.: SR/S1/OC-06/2011) for providing GPC facility for molecular weight characterization.Notes and references
- L. Yu, K. Dean and L. Li, Prog. Polym. Sci., 2006, 31, 576–602 CrossRef CAS PubMed.
- H. Y. Kweon, M. K. Yoo, K. Park, T. H. Kim, H. C. Lee and H. S. Lee, Biomaterials, 2003, 24, 801–808 CrossRef CAS.
- J. P. Jain, M. Sokolsky, N. Kumar and A. J. Domb, Polym. Rev., 2008, 48, 156–191 CrossRef CAS PubMed.
- M. Labet and W. Thielemans, Chem. Soc. Rev., 2009, 38, 3484–3504 RSC.
- M. Okada, Prog. Polym. Sci., 2002, 27, 87–133 CrossRef CAS.
- J. H. Khan, F. Schue and G. A. George, Polym. Int., 2009, 58, 296 CrossRef CAS PubMed.
- G. W. V. Cave, C. L. Raston and J. L. Scott, Chem. Commun., 2001, 2159 RSC.
- N. Nomura, T. Aoyama, R. Ishii and T. Kondo, Macromolecules, 2005, 38, 5363–5366 CrossRef CAS.
- Y. Takashima, Y. Nakayama, K. Watanabe, T. Itono, N. Ueyama, A. Nakamura, H. Yasuda and A. Harada, Macromolecules, 2002, 35, 7538–7544 CrossRef CAS.
- A. Kowalski, J. Libiszowski, T. Biela, M. Cypryk, A. Duda and S. Penczek, Macromolecules, 2005, 38, 8170–8176 CrossRef CAS.
- M. Vivas and J. Contreras, Eur. Polym. J., 2003, 39, 43–47 CrossRef CAS.
- H. J. Chuang, H. L. Chen, J. L. Ye, Z. Y. Chen, P. L. Huang, T. T. Liao, T. E. Tsai and C. C. Lin, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 696–707 CrossRef CAS PubMed.
- D. Deivasagayam and F. Peruch, Polymer, 2011, 52, 4686–4693 CrossRef CAS PubMed.
- K. C. Gupta and A. K. Sutar, Coord. Chem. Rev., 2008, 252, 1420–1450 CrossRef CAS PubMed.
- D. A. Atwood and M. J. Harvey, Chem. Rev., 2001, 101, 37–52 CrossRef CAS PubMed.
- H. S. Nalwa, Handbook of Low and High Dielectric Constant Materials and Their Applications, Academic Press, London, 1999 Search PubMed.
- Y. B. Cohen, Electroactive Polymer (EAP) Actuators as Artificial Muscles, SPIE Press, Bellingham, WA, 2004 Search PubMed.
- Q. M. Zhang, V. Bharti and X. Zhao, Science, 1998, 280, 2101–2104 CrossRef CAS.
- G. Lu, X. Li, H. Jiang and X. Mao, J. Appl. Polym. Sci., 1996, 62, 2193–2199 CrossRef CAS.
- C. W. Nan, Prog. Mater. Sci., 1993, 37, 1–116 CrossRef CAS.
- D. K. Das-Gupta, Ferroelectric Polymer and Ceramic–Polymer Composites, Trans Tech, Aedermannsdorf, Switzerland, 1994 Search PubMed.
- V. Tomer, E. Manias and C. A. Randall, J. Appl. Phys., 2011, 110, 044107 CrossRef PubMed.
- W. Prellier, M. P. Singh and P. Murugavel, J. Phys.: Condens. Matter, 2005, 17, 803–832 CrossRef.
- M. Fiebig, J. Phys. D: Appl. Phys., 2005, 38, 123–152 CrossRef.
- S. Roy, R. Chatterjee and S. B. Majumder, J. Appl. Phys., 2011, 110, 036101 CrossRef PubMed.
- A. V. Reddy, K. C. Sekhar, N. Dabra, A. Nautiyal, J. S. Hundal, N. P. Pathak and R. Nath, ISRN Mater. Sci., 2011, 5, 142968 Search PubMed.
- N. Ananthi, U. Balakrishnan and S. Velmathi, ARKIVOC, 2010, ix, 370 Search PubMed.
- M. Hayashi, Y. Miyamoto, T. Inoue and N. Oguni, J. Org. Chem., 1993, 58, 1515–1522 CrossRef CAS.
- M. Muneeswaran, P. Jegatheesan, M. Gopiraman, I. S. Kim and N. V. Giridharan, Appl. Phys. A, 2014, 114, 853–859 CrossRef CAS.
- A. Elzubair, C. N. Elias, J. C. M. Suarez, H. P. Lopes and M. V. B. Vieira, J. Dent., 2006, 34, 784–789 CrossRef CAS PubMed.
- J. Ren, T. Yu, H. Li, T. Ren and S. Yang, Polym. Compos., 2008, 29, 1145–1151 CrossRef CAS PubMed.
- J. R. Teague, R. Gerson and W. J. James, Solid State Commun., 1970, 8, 1073–1074 CrossRef CAS.
- X. X. Wang, S. H. Choy, X. G. Tang and H. L. W. Chan, J. Appl. Phys., 2005, 97, 104101–104104 CrossRef PubMed.
- B. G. Soares, M. E. Leyva, G. M. O. Barra and D. Khastgir, Eur. Polym. J., 2006, 42, 676–686 CrossRef CAS PubMed.
- P. B. Macedo, C. T. Moynihan and R. Bose, Phys. Chem. Glasses, 1972, 13, 171–179 CAS.
- P. S. Das, P. K. Chakraborty, B. Behera and R. N. P. Choudhary, Electrical properties of Li2BiV5O15 ceramics, Phys. B, 2007, 395, 98–103 CrossRef CAS PubMed.
- Z. Peng, Q. Chen, J. Wu, D. Liu, D. Xiao and J. Zhu, J. Alloys Compd., 2012, 541, 310–316 CrossRef CAS PubMed.
- P. Smyth, Dielectric Behavior and Structure, McGraw-Hill, New York, 1965 Search PubMed.
- M. Barsoum, Fundamental of Ceramics, McGraw-Hill, New York, 1997 Search PubMed.
- S. Zhang, L. Wang, Y. Chen, D. Wang, Y. Yao and Y. Ma, J. Appl. Phys., 2012, 111, 074105 CrossRef PubMed.
- W. Cai, C. L. Fu, Z. B. Lin and X. L. Deng, Ceram. Int., 2011, 37, 3643–3650 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |