Recent development of plant products with anti-glycation activity: a review
Abstract
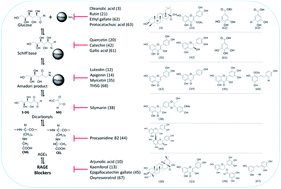
Diabetes mellitus (DM) is an endocrine disorder characterized by chronic hyperglycemia, which results from an absolute or a relative deficiency of insulin or resistance to insulin. Hyperglycemia is increasingly linked to the pathogenesis of diabetic complications in individuals with long-duration diabetes. One of the inevitable consequences of hyperglycemia is the enhanced accumulation of advanced glycation end-products (AGEs), which are implicated in the pathogenesis of diabetes. Various natural products and their active constituents have reportedly been used for the treatment of diabetes and its complications. Some of these molecules are known to have anti-glycation activity. The search for novel anti-glycation agents from various sources is gaining a lot of importance. Attention has especially been focused on plants with an ethnopharmacological background and also on plants rich in triterpenoids and phenolics, which generally exhibit antioxidant and anti-glycation effects. Plant extracts or compounds obtained from them that possess both antioxidant and anti-glycation activities might have great therapeutic potential for treating diabetic complications. This review highlights the anti-glycation activities of phytochemicals, which will aid in the identification of lead molecules for the development of new anti-glycation drugs.

 Please wait while we load your content...
Please wait while we load your content...