Synergistic effect of the valence bond environment and exposed crystal facets of the TiO2/SnS2 heterojunction for achieving enhanced electrocatalytic oxygen evolution†
Abstract
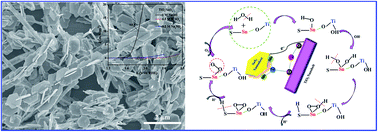
Cost-effective and high-performance electrocatalysts are vital factors that affect the development of the oxygen evolution reaction (OER). Herein, SnS2 nanosheets (SnS2 NSs)-modified TiO2 nanobelts (TiO2 NBs) (TiO2/SnS2) electrocatalyst with significant enhancement of the OER activity was successfully synthesized by the hydrothermal method. This was achieved by taking advantage of the fast radial transfer of the one-dimensional (1-D) TiO2 NBs, large areas of contact with the electrolyte and the stability of the two-dimensional (2-D) SnS2 NSs. The experimental results indicate that the change in the valence bond environment due to the formation of the heterojunction and the independent exposed crystal facets could distinctly reduce the charge-transfer resistance and the adsorption barrier of the H2O molecules in electrocatalytic water splitting, which are favourable for improving the OER activity. The synergistic effect of the valence bond environment and exposed crystal facets of the TiO2/SnS2 heterojunction catalyst have led to remarkable OER performance with an overpotential of 570 mV at the current density of 10 mA cm−2, a low Tafel slope of 107 mV dec−1 and long-time durability in alkaline solution. This non-precious TiO2/SnS2 heterojunction catalyst was designed based on the concept of combining valance factors and crystal facet engineering, which sheds light on a new pathway for the design of new, promising and highly efficient OER electrocatalysts.


 Please wait while we load your content...
Please wait while we load your content...