Relative sensitivities of TDAR, cytokine production, and immunophenotyping assays in immunotoxicity assessment
Abstract
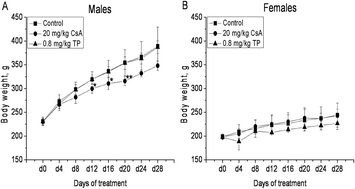
Additional immunotoxicity tests as part of a repeat-dose toxicity study have been recommended to improve the quality of immunotoxicity assessments. Present immunotoxicity test guidelines list many tests without concreteness of methods or principles for their use. Due to the different sensitivity and specificity of each assay, numerous different test batteries can produce diverse results and make difficult inter-laboratory comparisons. To help resolve these problems and identify some of the more sensitive tests that might be used, we established immunotoxicity in a rat model using two well-known immunosuppressants and then compared the sensitivity of three important immunotoxicity tests, i.e., measures of T-cell dependent antibody responses (TDAR), ex vivo cytokine production, and immunophenotyping. The results showed that after treatment with cyclosporin A (CsA; 20 mg kg−1 d−1) or triptolide (TP; 0.8 mg kg−1 d−1), production of interleukin (IL)-2, interferon (IFN)-γ, tumor necrosis factor (TNF)-α, and the antibody to keyhole limpet hemocyanin (KLH) were significantly inhibited; in contrast, there were only weak or sporadic changes to host histopathology and hematology. With regard to phenotyping, no significant changes were noted in the ratios of CD4+ : CD8+ cells in the treated hosts. Taken together, the results here suggest to us that cytokine production and the KLH TDAR assay are very sensitive parameters to define an agent as immunotoxic, while immunophenotyping and histopathology – while being more expensive/time-consuming – appear to be less sensitive options for an individual assessing the immunotoxic potential of a given test agent.

 Please wait while we load your content...
Please wait while we load your content...