Open Access Article
Open Access ArticleEvaluating the effects of nacre on human skin and scar cells in culture†
Vipul
Agarwal
a,
Edwin S.
Tjandra
a,
K. Swaminathan
Iyer
a,
Barry
Humfrey
b,
Mark
Fear
c,
Fiona M.
Wood
c,
Sarah
Dunlop
d and
Colin L.
Raston
*e
aSchool of Chemistry and Biochemistry, The University of Western Australia, Crawley, Australia
bPearl Technology Pty Ltd, Geraldton, Australia
cBurn Injury Research Unit, School of Surgery, The University of Western Australia, Crawley, Australia
dSchool of Animal Biology, The University of Western Australia, Crawley, Australia
eSchool of Chemical and Physical Sciences, Flinders University, Bedford Park, Australia. E-mail: colin.raston@flinders.edu.au; Tel: +61 82017958
First published on 2nd May 2014
Abstract
Pearl nacre, a biomineralisation product of molluscs, has growing applications in cosmetics, as well as dental and bone restoration, yet a systematic evaluation of its biosafety is lacking. Here, we assessed the biocompatibility of nacre with two human primary dermal fibroblast cell cultures and an immortalised epidermal cell line and found no adverse effects.
There are three main types of pearl oysters of the genus Pinctada: the “Akoya” pearl oyster called Pinctada fucata, the “Golden lipped” oyster Pinctada maxima and the “Black lipped” oyster named Pinctada margaritifera. Mollusc shells are mainly made up of two layers of calcium carbonate, comprising an outer layer of calcite and an inner layer of aragonite. Nacre (mother of pearl) in all oyster shells is a calcified structure that forms the lustrous inner layer. It is mainly composed of aragonite (∼95–97%) tablets oriented in multiple layers, each surrounded by organic matrix.1,2 This organic matrix makes up ∼5% of the nacre composition and is mainly comprised of polysaccharides and proteins.3 According to a European Commission report published in 2007 the cosmetic and toiletries industry in the EU, Japan, China and the US had a total market value of €136.2 billion.4 The cosmetics industry maintains its edge by constantly developing novel topical skin treatments. A popular example is the use of all-natural or organic ingredients, such as fruit and plant extracts to offer wrinkle relief that mimics the painful and potentially dangerous side effects associated with invasive chemical remedies.5 Clinically, topical treatments containing, for example, aloe vera, vitamin C, corticosteroids and tacrolimus are used with the aim of minimizing scarring.6 Recently, there has been interest in the cosmetics industry in the use of nacre as a key ingredient.7 Most of the formulations are reported to either use powdered pearl shell or powdered nacreous layer shell. Powdered shell and powdered nacre comprises of both organic and inorganic components. It is reported that nacre stores in its mineral-based organic structure a variety of bioactive molecules. Efficacy of this water soluble matrix (WSM) has been tested in a porcine burn injury model.8 WSM was obtained by suspending powdered nacre in ultra-pure water and collecting the supernatant via precipitation of insoluble components by centrifugation. It was concluded that the active mineral based organic component has beneficial effects on the skin with enhanced wound healing.8,9
Nacre has also attracted attention for its potential in supporting bone grafting and bone regeneration. In culture under physiological conditions, nacre can transform to hydroxyapatite, the phosphorous rich main constituent of the mammalian bone framework.10,11 Nacre and its WSM can also aid in osteogenic regeneration.9,12–17 High phosphorous rich domains have been described at the interface between bone and implants made from Margaritifera shells which are biocompatible, biodegradable and osteoconductive and thus are thought to promote bone formation.18 Furthermore, nacre powder has been used as an implantable material for reconstruction and regeneration of maxillary alveolar ridge bone in humans.19 In this example, the implanted nacre dissolves gradually and is eventually replaced by the mature lamellar bone suggesting that the nacre acts as a biocompatible substrate for bone replacement.19 The water soluble components of the crushed nacre have also been investigated for their potential in bone regeneration in a similar vein.20,21 Lee et al., demonstrated the wound healing potential of WSM component in a deep burn porcine skin model and showed enhanced collagen secretion and deposition at the injury site resulting in enhanced healing.8 In another in vivo study using a rat skin incisional injury model, powdered nacre was implanted between the epidermis and dermis at the incisional site, with an aim of studying the effect of nacre on the synthesis of certain constituents of the dermal extracellular matrix. It was concluded that implanted nacre increased collagen synthesis by dermal fibroblasts.22 While extensive investigations have been carried out in bone, the evaluation of the biocompatibility of nacre with human skin cells is lacking. Thus the growing number of cosmetic formulations in the market with nacre as a key ingredient7,23,24 clearly warrants a thorough assessment with human skin cells. Since scars are also common, and contain cells with a phenotype distinct from normal skin,25 it is also important to test potential cosmetic ingredients with both cell types.
In the present study, we use nacre from the inner calcified layer of the shell of Pinctada margaritifera and report the in vitro toxicity assessment of the material on three cell types representing both epidermal and dermal layers of human skin. These were HaCaT cells, a human derived immortalised keratinocytes cell line, primary human dermal skin fibroblasts (HDF) and primary human scar fibroblasts (HSF).
Nacre used in the study was gently scraped26,27 from the inner layer of the shell to avoid the post processing required in the case of powdered shell. SEM images (Fig. 1) confirmed that the nacre was composed of pseudo-hexagonal shaped aragonite tablets which have basal plane dimensions of 2–6 μm, and a thickness of 300–400 nm.28 This structure is characteristic of previously reported nacre, which is a composite material consisting of alternating layers of mineral tablets separated by thin layers of biomacromolecular “glue”.29,30
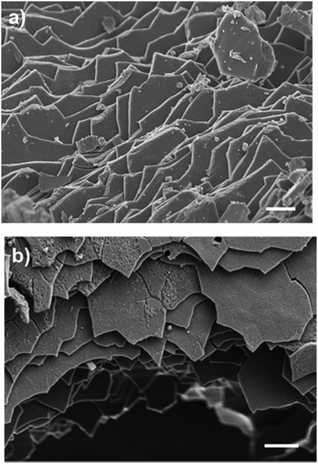 | ||
| Fig. 1 Top view of the scrapped nacre, imaged using scanning electron microscopy (SEM). Scale bar (a) 1 μM and (b) 2 μM respectively. Sample was coated with 4 nm platinum prior to imaging. | ||
To test the cytotoxicity of nacre, a live/dead assay was carried out (see ESI S1.4†). Cells were incubated with nacre in culture media at physiological conditions for 24 h to 72 h and were then stained for viability using calcein AM/ethidium bromide I solutions. Viable cells fluoresce green through the reaction of calcein AM with intracellular esterase, whereas non-viable cells fluoresce red due to the diffusion of ethidium homodimer across damaged cell membranes and binding with nucleic acids.
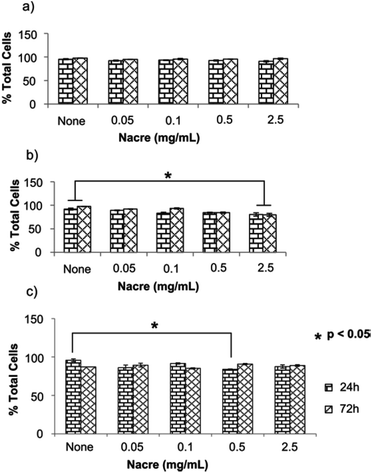
Fig. 2 shows live cells as the percentage of the total cells in human primary dermal skin fibroblast (HDF), human primary scar fibroblast (HSF) and human derived immortalised HaCaT cell cultures when exposed to various concentrations of nacre for 24 h and 72 h. Cytotoxicity of nacre was not observed for any of the concentrations examined in HDF cells (Fig. 2a). However, interestingly at a concentration of 2.5 mg ml−1 of nacre (highest concentration tested) there was a significant reduction in viability at both 24 and 72 hours in the HSF cells (Fig. 2b). This underlines the importance of testing both scar and normal skin cell types for cosmetic application. Toxicity was also observed at a concentration of 0.5 mg ml−1 in HaCaT cells (Fig. 2c), although this was only observed at 24 hours and was no longer present at the 72 hour time point. These results are in line with the results obtained previously where constituents of nacre were shown to promote wound healing in a rat model22 and deep burn porcine skin.8 In both these studies, nacre has been shown to promote the recruitment of fibroblasts for restoration and coverage of the injury site while showing no apparent signs of cytotoxicity. It has also been shown to promote bone formation when implanted in the femur of sheep with midshaft hemidiaphysis resection of their femur in vivo reiterating the non-cytotoxic advantage of nacre.31
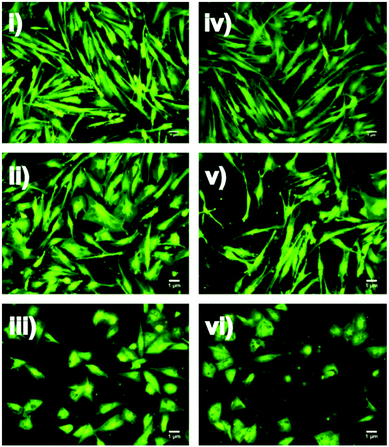
Fibroblasts have been reported to undergo morphological changes from dendritic to stellate shapes upon exposure to external cues caused by changes in actin polarisation and adhesion.32,33 Cell morphology in fibroblasts is known to be influenced by cytokines such as transforming growth factor β which can potentially induce polymerisation of globular to filamentous actin.34 Fibroblast morphology can also be modulated by extracellular matrix architecture during wound healing via cell–matrix interaction.32 Such morphological change has been observed in cells undergoing oxidative stress.35,36 In our study, we found similar changes in fibroblast morphology for both HDF and HSF cells at the highest concentration of nacre of 2.5 mg mL−1 (Fig. 3). Similar altered morphology was also observed for HaCaT cells (see ESI Fig. S1†). It could be postulated that the high concentration of nacre induces cellular stress, resulting in changes in the actin cytoskeleton and a more stellate morphology (Fig. 3i, iii, ii and iv). Cell area was calculated from the fluorescent images shown in Fig. 3 using Image J software.37 It was found that both HDF and HSF had significantly larger cell areas (p < 0.05) when treated with 2.5 mg mL−1 of nacre (HDF: 2.79 ± 0.13 μm and HSF: 3.0 ± 0.19 μm respectively) as compared to the non-treated controls (HDF: 1.56 ± 0.08 μm and HSF: 1.54 ± 0.10 μm respectively) (see ESI Fig. S2†).
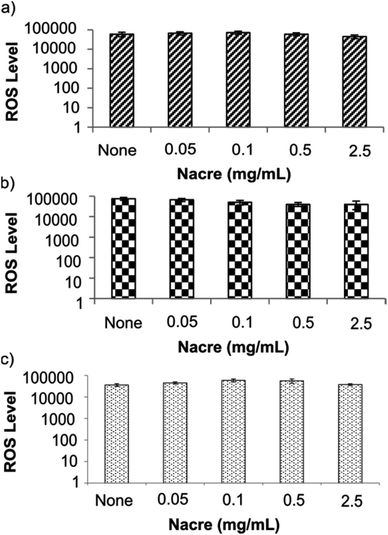
Altered fibroblast morphology has been thought to occur in response to various factors including aging,38 strength of the extracellular matrix39 or other etiologies that induce mechanical stress on the cell. Changes in morphology also commonly indicate oxidative as well as mechanical stress.39 Therefore, we explored whether the morphological changes and increase in cell area at the highest concentration of nacre in culture was a result of, or induced oxidative stress in, the cells. Oxidative stress was tested using the cell permeable fluorogenic probe 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA). DCFH-DA is taken up by cells and is deacetylated by cellular esterases to non-fluorescent 2′,7′-dichlorodihydrofluorescein (DCFH) which is rapidly oxidised by reactive oxygen species (ROS) to highly fluorescent 2′,7′-dichlorodihydrofluorescein (DCF). The fluorescent intensity is proportional to the ROS levels within the cytosol (see ESI S1.5†). Cell responsiveness to the assay was carried out by stressing the cells with the H2O2 solution provided in the kit which was also used to generate the calibration curve (see ESI Fig. S3†). No changes in levels of reactive oxygen species were observed in any cell type at any concentration of nacre (Fig. 4). This is important as oxidative stress is known to be a significant contributor to skin damage and excessive scarring in previous studies.40 It has been known that cells alter their morphology depending on their environment.41,42 It is therefore hypothesized that the altered fibroblast morphology in the present case is mainly due to the regulation of cell motility through geometrical constraint in the presence of nacre. Indeed, it has been previously reported that when cells probe their physical surroundings, they acquire mechanical information or signals that help to determine the direction of migration, with a consequential change in cell morphology.43
Conclusions
We have established the biocompatibility of nacre using three human cell types representing the two primary layers of human skin, using immortalised keratinocytes from the epidermal layer and two primary human dermal cell cultures. The nacre used in the present study showed limited cytotoxicity at high concentrations in scar derived cells, with the morphology of the cells significantly changed by exposure at such concentrations of nacre. No apparent oxidative stress was evident in any of the cell types. Overall, the data support the use of low concentrations of nacre in aesthetic formulations, with the potential for high concentrations to cause changes in skin and/or scar cells which may have impact on efficacy.The authors would like to thank the Australian Research Council and Pearl Technologies for funding the work under the grant number LP100100812. The authors would also like to acknowledge the Australian Microscopy & Microanalysis Research Facility at the Centre for Microscopy, Characterization & Analysis, The University of Western Australia, funded by the University, State and Commonwealth Governments.
Notes and references
- J. H. E. Cartwright and A. G. Checa, J. R. Soc. Interface, 2007, 4, 491–504 CrossRef CAS PubMed.
- F. Nudelman, E. Shimoni, E. Klein, M. Rousseau, X. Bourrat, E. Lopez, L. Addadi and S. Weiner, J. Struct. Biol., 2008, 162, 290–300 CrossRef CAS PubMed.
- Y. Oaki and H. Imai, Angew. Chem., Int. Ed., 2005, 44, 6571–6575 CrossRef CAS PubMed.
- D. G. f. E. a. I. European Commission, A Study of the European Cosmetics Industry, Report, European Commission, 2007.
- P. M. Reddy, M. Gobinath, K. M. Rao, P. Venugopalaiah and N. Reena, Int. J. Adv. Pharm. Nanotechnol., 2011, 1, 121–139 Search PubMed.
- J. M. Zurada, D. Kriegel and I. C. Davis, J. Am. Acad. Dermatol., 2006, 55, 1024–1031 CrossRef PubMed.
- Pearlcium, Genesis Framework – WordPress, vol. 2013, p. US.
- K. Lee, H. Kim, J. Kim, Y. Chung, T. Lee, H.-S. Lim, J.-H. Lim, T. Kim, J. Bae, C.-H. Woo, K.-J. Kim and D. Jeong, Mol. Biol. Rep., 2012, 39, 3211–3218 CrossRef CAS PubMed.
- M. J. Almeida, C. Milet, J. Peduzzi, L. Pereira, J. Haigle, M. Barthélemy and E. Lopez, J. Exp. Zool., 2000, 288, 327–334 CrossRef CAS.
- Y. Guo and Y. Zhou, J. Biomed. Mater. Res., Part A, 2008, 86, 510–521 CrossRef PubMed.
- P. Westbroek and F. Marin, Nature, 1998, 392, 861–862 CrossRef CAS PubMed.
- M. Lamghari, M. J. Almeida, S. Berland, H. Huet, A. Laurent, C. Milet and E. Lopez, Bone, 1999, 25, 91S–94S CrossRef CAS.
- M. Lamghari, H. Huet, A. Laurent, S. Berland and E. Lopez, Biomaterials, 1999, 20, 2107–2114 CrossRef CAS.
- M. Lamghari, S. Berland, A. Laurent, H. Huet and E. Lopez, Biomaterials, 2001, 22, 555–562 CrossRef CAS.
- F. Moutahir-belqasmi, N. Balmain, M. Lieberrher, S. Borzeix, S. Berland, M. Barthelemy, J. Peduzzi, C. Milet and E. Lopez, J. Mater. Sci.: Mater. Med., 2001, 12, 1–6 CrossRef CAS.
- D. Duplat, A. Chabadel, M. Gallet, S. Berland, L. Bédouet, M. Rousseau, S. Kamel, C. Milet, P. Jurdic, M. Brazier and E. Lopez, Biomaterials, 2007, 28, 2155–2162 CrossRef CAS PubMed.
- C. Silve, E. Lopez, B. Vidal, D. Smith, S. Camprasse, G. Camprasse and G. Couly, Calcif. Tissue Int., 1992, 51, 363–369 CrossRef CAS.
- H. Liao, H. Mutvei, M. Sjöström, L. Hammarström and J. Li, Biomaterials, 2000, 21, 457–468 CrossRef CAS.
- G. Atlan, N. Balmain, S. Berland, B. Vidal and É. Lopez, C. R. l'Academie. Sci., Ser. III, 1997, 320, 253–258 CAS.
- L. Pereira Mouriès, M.-J. Almeida, C. Milet, S. Berland and E. Lopez, Comp. Biochem. Physiol., Part B: Biochem. Mol. Biol., 2002, 132, 217–229 CrossRef.
- M. Rousseau, H. Boulzaguet, J. Biagianti, D. Duplat, C. Milet, E. Lopez and L. Bédouet, J. Biomed. Mater. Res., Part A, 2008, 85, 487–497 CrossRef PubMed.
- E. Lopez, A. L. Faou, S. Borzeix and S. Berland, Tissue Cell, 2000, 32, 95–101 CrossRef CAS.
- E. Lopez and A. E. Chemouni, US Pat, US10/089,982, 2005 Search PubMed.
- D. M. DeLaRosa, US Pat, US2008/0199533 A1, 2008 Search PubMed.
- C. Chipev and M. Simon, BMC Dermatol., 2002, 2, 13 CrossRef PubMed.
- M. Norizuki and T. Samata, Mar. Biotechnol., 2008, 10, 234–241 CrossRef CAS PubMed.
- C. Ma, C. Zhang, Y. Nie, L. Xie and R. Zhang, Tsinghua Sci. Technol., 2005, 10, 499–503 CrossRef CAS.
- K. S. Katti, B. Mohanty and D. R. Katti, J. Mater. Res., 2006, 21, 1237–1242 CrossRef CAS.
- R. A. Boulos, C. Harnagea, X. Duan, R. N. Lamb, F. Rosei and C. L. Raston, RSC Adv., 2013, 3, 3284–3290 RSC.
- E. S. Tjandra, R. A. Boulos, P. C. Duncan and C. L. Raston, CrystEngComm, 2013, 15, 6896–6900 RSC.
- S. Berland, O. Delattre, S. Borzeix, Y. Catonné and E. Lopez, Biomaterials, 2005, 26, 2767–2773 CrossRef CAS PubMed.
- E. Tamariz and F. Grinnell, Mol. Biol. Cell, 2002, 13, 3915–3929 CrossRef CAS PubMed.
- Q. M. Chen, V. C. Tu, J. Catania, M. Burton, O. Toussaint and T. Dilley, J. Cell Sci., 2000, 113, 4087–4097 CAS.
- A. Moustakas and C. Stournaras, J. Cell Sci., 1999, 112, 1169–1179 CAS.
- W. Malorni, F. Iosi, F. Mirabelli and G. Bellomo, Chem. Biol. Interact., 1991, 80, 217–236 CrossRef CAS.
- G. Bellomo, F. Mirabelli, M. Vairetti, F. Iosi and W. Malorni, J. Cell. Physiol., 1990, 143, 118–128 CrossRef CAS PubMed.
- T. J. Collins, BioTechniques, 2007, 43, S25–S30 CrossRef PubMed.
- J. Varani, M. K. Dame, L. Rittie, S. E. G. Fligiel, S. Kang, G. J. Fisher and J. J. Voorhees, Am. J. Pathol., 2006, 168, 1861–1868 CrossRef CAS PubMed.
- G. J. Fisher, T. Quan, T. Purohit, Y. Shao, M. K. Cho, T. He, J. Varani, S. Kang and J. J. Voorhees, Am. J. Pathol., 2009, 174, 101–114 CrossRef CAS PubMed.
- R. E. Barrow and M. R. K. Dasu, J. Surg. Res., 2005, 126, 59–65 CrossRef CAS PubMed.
- M. Dalby, M. Riehle, H. Johnstone, S. Affrossman and A. Curtis, Tissue Eng., 2002, 8, 1099–1108 CrossRef CAS PubMed.
- K. Burridge and M. Chrzanowska-Wodnicka, Annu. Rev. Cell Dev. Biol., 1996, 12, 463–519 CrossRef CAS PubMed.
- R. J. Petrie, A. D. Doyle and K. M. Yamada, Nat. Rev. Mol. Cell Biol., 2009, 10, 538–549 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4tx00004h |
| This journal is © The Royal Society of Chemistry 2014 |