Effects of co-exposure to arsenic and dichlorvos on glutathione metabolism, neurological, hepatic variables and tissue histopathology in rats
S. J. S.
Flora
*,
Nidhi
Dwivedi
,
Utsab
Deb
,
Pramod
Kushwaha
and
Vinay
Lomash
Division of Regulatory Toxicology, Defence Research and Development Establishment, Jhansi Road, Gwalior-474 002, India. E-mail: sjsflora@hotmail.com; sjsflora@drde.drdo.in; Fax: +91 751 2341148; Tel: +91 751 2378196
First published on 24th July 2013
Abstract
The individual toxic effects of arsenic and organophosphate pesticides are known but there is a lack of data on their combined effects. The present study investigates the toxic effects following combined exposure to organophosphate and arsenic on: (i) alterations in brain biogenic amines, (ii) oxidative stress and its correlation with glutathione linked enzymes, cell death and histopathological observations, and (iii) arsenic concentration in soft tissues. Rats were exposed to arsenic (1 mg kg−1 body weight, orally) and dichlorvos (4 mg kg−1 body weight, subcutaneously) either individually or in combination for 16 weeks. Arsenic alone and in combination with DDVP led to a significant increase in reactive oxygen species (ROS), thiobarbituric acid reactive substance (TBARS), glutathione peroxidase (GPx) and glutathione-S-transferase (GST) activities accompanied by a decreased glutathione reductase, reduced and oxidized glutathione (GSH and GSSG) levels in tissues. Arsenic and DDVP also produced a significant depletion in brain biogenic amines; however, acetylcholinesterase (AChE) activity increased moderately on arsenic exposure. Arsenic and DDVP co-exposure exhibited synergism in the case of ROS while no such effect was noted in the case of TBARS. Interestingly, combined exposure to arsenic and DDVP resulted in more pronounced toxic effects compared to their individual effects based on various biochemical variables, cell death (TUNEL assay) and histopathological observations. Interestingly, brain arsenic levels decreased on co-exposure to arsenic and DDVP accompanied with prominent elevation of liver arsenic content in co-exposed groups. The present study thus provides some interesting observations on the interaction between arsenic and DDVP including (i) co-exposure to arsenic and DDVP might lead to significant oxidative stress and (ii) their co-exposure produced synergistic effects on some liver variables but some antagonistic effects on the brain.
Introduction
Arsenic is a well known water contaminant widely distributed around the world. Humans are exposed to this metalloid through well water, contaminated soil and through occupational exposure that may cause neuropathy, skin lesions, vascular lesions and cancer upon prolonged exposure.1,2 Groundwater contamination with arsenic in India particularly West Bengal is reported to be the largest arsenic calamity in the world and has now reached worldwide as per the World Health Organization drinking water guidelines.3–5 Several studies indicate that ingestion of arsenic can result in neural injury with symptoms such as headache, lethargy, mental confusion, hallucination, seizures and coma.6 Meanwhile, arsenic accumulates in the liver and induces hepatotoxicity changes like cirrhosis, portal hypertension, fatty degeneration and primary hepatic neoplasia.7,8Organophosphate poisoning is common due to both occupational and intentional means. Efforts to increase the quality and quantity of crop yields in many countries have led to a significant rise in the use of pesticides. Concerns have been raised due to their persistence in the environmental matrices such as air and soil and their presence in surface water runoff and due to their toxic effects. The uncontrolled use of these pesticides in agriculture and public health has increased the scope of the ecological imbalance. It is reported that organophosphate pesticides are neurotoxic in nature by acting as inhibitors of neuronal cholinesterase (ChE).9 The main function of AChE is rapid hydrolysis of the neurotransmitter acetylcholine at cholinergic synapses, and it is one of the fastest enzymes known. This inhibition leads to an accumulation of ACh at synapses and neuromuscular junctions (NMJ) causing over-stimulation and subsequent dysfunction of transmission of impulses in the central, peripheral, and autonomic nervous systems.10,11 Dichlorvos (DDVP) is being widely and effectively used throughout the world with applications in agriculture and horticulture for controlling insects in crops.
Oxidative stress is considered to be a potential mechanism for both arsenic and organophosphate pesticides. Arsenic exposure causes alterations in neurotransmitter levels such as cholinergic, monoaminergic, glutaminergic and GABAergic.12–14 Arsenic accumulation in the nervous system leads to neurotoxicity via the formation of reactive oxygen species (ROS) as the brain is more vulnerable to oxidative damage because of high oxygen utilization, the presence of unsaturated fatty acids and decreased activity of detoxifying enzymes.15 In general, pesticides have been shown to induce apoptosis in neural and immune cells via the mitochondria mediated pathway16,17 and alter the cellular redox balance by different mechanisms including (i) generation of reactive oxygen species (ROS); (ii) depletion of antioxidant defenses; and (iii) the impairment of antioxidant enzyme function.18
Most of the past studies focused on the individual toxic effects of a chemical, however, there is every possibility that humans and animals can be exposed to and are at risk from a mixture of chemicals arising from various sources.19 Metal and organophosphate pesticides are among the major contaminants in the environment and because of their persistence and higher toxicity, these toxicants may cause serious health problems. The adverse effects produced by arsenic or organophosphate pesticides alone in humans and animals are well documented but there is very little scientific knowledge known about the health problems arising from their combined exposure. In case of combined or multiple exposure, interactions are of special importance. On the basis of our prior study, there were some interesting observations which suggested that co-exposure to arsenic and DDVP may not necessarily lead to synergistic effects.20
The present study was designed based on the hypothesis that in view of co-exposure, one chemical may alter the biochemical metabolism of another by influencing the kinetics, resulting in a response which may be additive or reductive in nature. In some cases, the effect may be potentiating due to the interference exerted by one compound on the detoxification process of the other compound. Thus in this study, changes in neurological variables, particularly the levels of biogenic amines and acetylcholinesterase activity, glutathione linked enzymes and arsenic concentration in both brain and liver, were examined. We also assessed histopathological changes in the brain and liver supported by a TUNEL assay for cell death to delineate the possible mechanisms for the observed effects following co-exposure to arsenic and DDVP.
Material and methods
Chemicals and reagents
Dichlorvos; DDVP (Nuvan 76%) was obtained from Syngenta Chemicals (India) and sodium m-arsenite (NaAsO2, molecular weight 129.9) was procured from Sigma Chemical (USA). All other analytical laboratory chemicals and reagents were purchased from Merck (Germany), Sigma (USA) or BDH Chemicals (Mumbai, India). Ultra pure water, prepared by Millipore (New Delhi, India), was used throughout the experiment to avoid metal contamination and for the preparation of reagents and buffers, used for various biochemical assays in our study.Animals and treatments
Male Wistar rats (110–120 g) were obtained from the Defence Research and Development Establishment (DRDE) animal facility and prior to use, were acclimatized for 7 days in a 12 h light–dark cycle. The Institutional Animal Ethical Committee of DRDE, Gwalior, India, approved the protocols for the experiments. The animals were housed in stainless steel cages in an air-conditioned room with the temperature maintained at 25 ± 2 °C. Rats were allowed a standard chow diet (Ashirwad Feeds, Chandigarh, India; metal content of diet, in ppm dry weight: Cu 10.0, Zn 45.0, Mn 55.0, Co 5.0, Fe 75.0) throughout the experiment and waterad libitum. Forty animals were randomized into four groups of ten rats each and were treated as below for 16 weeks:Group I: normal (received normal water)
Group II: DDVP (4 mg kg−1, subcutaneously)
Group III: arsenic as sodium meta arsenite (1 mg kg−1, orally)
Group IV: DDVP + arsenic (as in group II and III respectively)
The animals were dosed once daily through the oral route for arsenic while it was subcutaneous for DDVP. Dosing was carried out 5 days per week at the same time between 1100 hours and 1200 hours. Distilled water was used as the vehicle for both arsenic and DDVP. The dissolution volume of arsenic (1 mg kg−1) and DDVP (4 mg kg−1) was calculated as per their doses. The single doses of each toxicant were chosen to produce a modest amount of injury and were based on earlier publications.21,22 We opted for a comparative smaller dose for DDVP than the one selected by Kaur et al.21 for the reason that duration of exposure in our study was 16 weeks while in case of Kaur et al. it was 12 weeks. The reasons for selecting two different modes of administration for the toxicants were (i) we wanted to avoid interaction between these toxicants in the gastrointestinal tract and (ii) absorption of pesticides is commonly through the skin while arsenic exposure is commonly through the oral route.
After 16 weeks, animals were euthanized and sacrificed by decapitation. We used 10 animals in each group in view of the fact that there were too many biochemical variables to be examined. Thus the sample size for each variable was 5. The brain and liver were removed, rinsed in cold saline, blotted, weighed and used for various biochemical variables and metal analysis. Half of the liver and brain from each rat was processed immediately for biochemical estimation and the remainder was stored at −20 °C before wet acid digestion with HNO3 for the determination of arsenic concentration. For the histopathological examinations, pieces of tissue were immediately transferred into 10% neutral buffered formalin.
Biochemical assays
The amount of ROS in the brain was measured using 2,7-dichlorofluorescein diacetate (DCFDA) which is converted into highly fluorescent DCF by cellular peroxides (including hydrogen peroxide). The assay was performed as described in the literature.23 Fluorescence was determined at 488 nm excitation and 525 nm emission wavelength, using a fluorescence plate reader (Perkin-Elmer, LS-55, United Kingdom). Tissue lipid peroxidation was measured.24 The amount of thiobarbituric acid reactive substances (TBARS) was calculated using a molar extinction coefficient of 1.56 × 105 M−1 cm−1.The brain and liver were assayed for GSH and GSSG content according to the method described in the literature, while tissue glutathione peroxidase activity (GPx) was also measured.25,26 Glutathione-S-transferase (GST) and glutathione reductase (GR) activities was determined following the reported procedure.27,28 A 10% brain homogenate (w/v) was prepared in 0.25 M sucrose for determining the activity of acetyl cholinesterase (AChE) using acetylthiocholine as substrate as described in the reported method.29
The frozen brain tissue samples were weighed and homogenized in acidified butanol. Dopamine (DA), norepinephrine (NE) and 5-hydroxytryptamine (5-HT) were estimated according to the procedure described in the literature.30
Histopathological examination
Liver and brain tissues were dissected out and fixed in 10% neutral buffered formalin solution. Tissues were processed in ascending concentrations of ethanol, cleared in toluene using an automatic tissue processor (Leica TP1020), further embedded in paraffin (Leica embedding station, EG1160) and serial sections (4–5 mm thickness for liver and 12 mm for brain) were cut with a Microm HM 360 microtome. Sections were stained by the routinely used Hematoxylin and Eosin (H&E) method using a standardized programme in a Leica autostainer XL and analyzed using a light microscope (Leica, DMLB).TUNEL assay for apoptosis
In situ apoptosis detection by TUNEL staining (Dead End™ Colorimetric TUNEL System, Catalogue no. G7131 and G7132; Promega, Madison, WI) was performed using the manufacturer’s protocol. DNA damage was identified as TUNEL positive cells, in tissue sections under a Leica DMLB microscope using Qwin Version 3 image analysis software (Leica Inc., Germany). Brain and liver sections pretreated with HCL were taken as positive controls for confirmation of TUNEL positive cells and DNA damage.Metal estimation
Arsenic concentration in the brain and liver were measured after wet acid digestion using a Microwave Digestion System (CEM, USA, model MDS-2100). Arsenic was estimated using a Hydride Vapor Generation System (Perkin Elmer model MHS-10) fitted with an atomic absorption spectrophotometer (AAS, Perkin Elmer model Analyst 100).31Statistical analysis
Data was presented as mean + SE. Data were also analyzed for statistical comparison using ANOVA followed by the Bonferroni test. A significance of P < 0.001 and P < 0.05 was considered significant. Based on the affected area in the microscopic field, histopathological and TUNEL variables were analyzed on an arbitrary scale of 0 to 3 [0—nil (absent); 1—minimal (1–10% area); 2—mild (11–25% area); 3—moderate (26–50% area)] and the results are presented in the form of a summary incidence table.Results
Effects on reactive oxygen species (ROS) and thiobarbituric acid reactive substance (TBARS)
Fig. 1 shows the individual and combined effects of arsenic and dichlorvos (DDVP) on ROS and TBARS levels in liver and brain tissues. Tissue ROS increased significantly on arsenic exposure, individually and in combination with DDVP. On the other hand, DDVP had only a marginal effect on ROS. Brain TBARS increased significantly on arsenic and DDVP exposures compared to the normal and co-exposed group. Significant change was observed in the liver TBARS level in the animals receiving DDVP. Arsenic alone produced only a marginal increase in the liver TBARS level.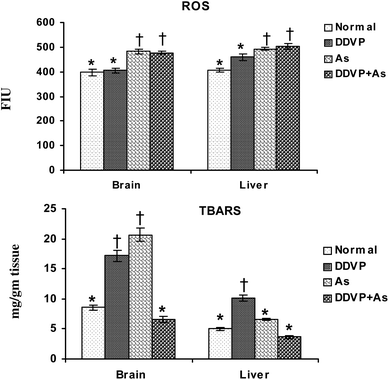 | ||
| Fig. 1 Effect of individual and combined exposure to As and DDVP on ROS and TBARS in brain and liver. Abbreviations and units used: ROS: reactive oxygen species such as FIU; TBARS: thiobarbituric reactive substances as mg g−1 tissue. Values are mean ± SE; n = 5. *,†Differences between values with matching symbol notations within each bar are not statistically significant at 5% level of probability. | ||
Effects on reduced glutathione (GSH) and oxidized glutathione (GSSG) levels
Brain GSH levels decreased significantly in all the exposed groups (Fig. 2), while brain GSSG levels increased significantly in the DDVP + As co-exposed group compared to the control animals. No significant change in hepatic GSH and GSSG levels was noted in any of the exposed groups except for a marginal non-significant increase in hepatic GSH level on arsenic exposure.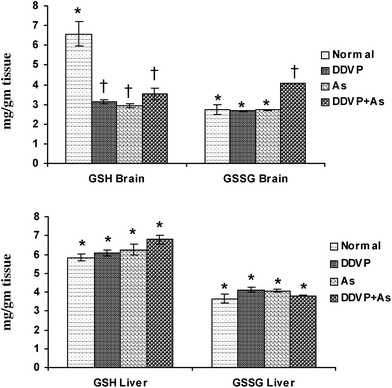 | ||
| Fig. 2 Effect of individual and combined exposure to As and DDVP on GSH and GSSG in brain and liver. Abbreviations and units used: GSH: reduced glutathione as mg g−1 tissue; GSSG: oxidized glutathione as mg g−1 tissue. Values are mean ± SE; n = 5, *,†Differences between values with matching symbol notations within each bar are not statistically significant at 5% level of probability. | ||
Effects on glutathione peroxidase (GPx), glutathione reductase (GR) and glutathione-S-transferase (GST) activity
The individual and combined effects of arsenic and DDVP on GPx, GR and GST activities in the brain and liver are presented in Fig. 3. A significant increase in brain GPx activity was observed in the DDVP exposed group but the change was more pronounced in the DDVP + As co-exposed animals compared to the controls. Liver GPx activity remained unchanged in all the exposed groups, while brain GR activity decreased significantly in the arsenic exposed group. DDVP and arsenic individually produced a significant decrease in hepatic GR activity, while during co-exposure, the activity remained unaltered. Brain GST activity showed a significant increase in the DDVP + As co-exposed group compared to the other individually exposed groups. Hepatic GST activity increased significantly in the arsenic alone and arsenic + DDVP co-exposed animals. The most pronounced increase was seen in the arsenic alone exposed group compared to the other groups.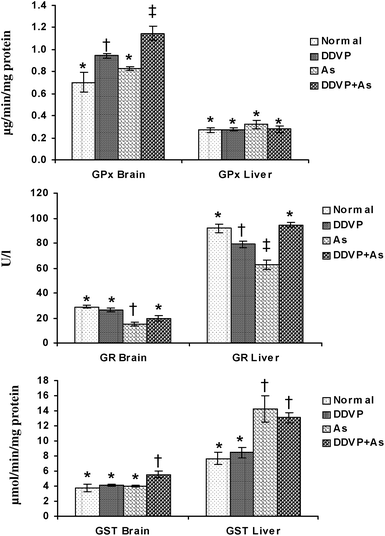 | ||
| Fig. 3 Effects of individual and combined exposure to As and DDVP on GPx, GR and GST in brain and liver. Abbreviations and units used: GPx: glutathione peroxidase as μg min−1 mg−1protein; GR: glutathione reductase as U l−1; glutathione-S-transferase as μmol min−1 mg−1protein. Values are mean ± SE; n = 5. *,†,‡Differences between values with matching symbol notations within each bar are not statistically significant at 5% level of probability. | ||
Effects on brain neurological variables
Brain biogenic amines (NE, DA, 5-HT) and AChE activity were studied in arsenic, DDVP and arsenic + DDVP co-exposed animals and the data are presented in Fig. 4. NE and DA levels decreased significantly in the arsenic and DDVP alone exposed groups compared to the controls. While there was a marked decrease in NE and DA levels following arsenic or DDVP exposure, the effects were less pronounced in the DDVP + As co-exposed group. 5-HT levels remained unaltered on exposure to arsenic and DDVP exposure.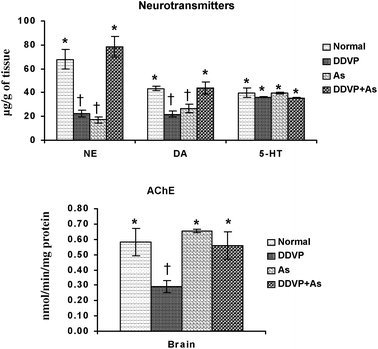 | ||
| Fig. 4 Effects of individual and combined exposure to As and DDVP on neurotransmitter levels and AChE activity in brain. Abbreviations and units used: NE: norepinephrine as μg g−1 of tissue; DA: dopamine as μg g−1 of tissue; 5-HT: 5-hydroxytryptamine as μg g−1 of tissue; AChE: acetyl cholinesterase as nmol min−1 mg−1protein. Values are mean ± SE; n = 5. *,†Differences between values with matching symbol notations within each bar are not statistically significant at 5% level of probability. | ||
The brain AChE activity showed an insignificant increase on arsenic exposure. The decrease was more pronounced in DDVP exposed animals compared to the other exposed groups. Interestingly the effects on AChE were relatively less pronounced in the As + DDVP co-exposed animals in comparison with their individual effect (Fig. 4).
Effects on tissue arsenic concentration
Tissue arsenic concentration increased in both the arsenic alone and arsenic + DDVP co-exposed groups. There was a small more pronounced increase in liver arsenic concentration in animals co-exposed to As + DDVP compared to arsenic alone but the increase was non-significant. Likewise the brain arsenic concentration in DDVP + As co-exposed animals was decreased marginally (non-significantly) compared to arsenic alone. Interestingly, brain arsenic concentration was non-significant in the DDVP + As exposed animals compared to arsenic exposed animals (Fig. 5).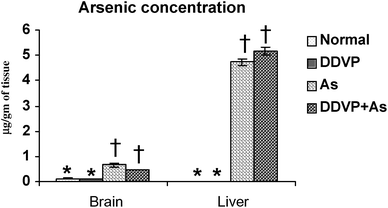 | ||
| Fig. 5 Effect of individual and combined exposure to As and DDVP on arsenic concentration in brain and liver. Units: arsenic in brain and liver as μg g−1 of tissue. Values are mean ± SE; n = 5. *,†Differences between values with matching symbol notations within each bar are not statistically significant at 5% level of probability. | ||
Histopathological observations
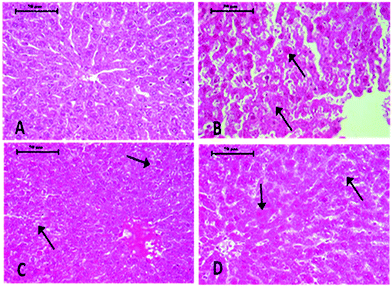
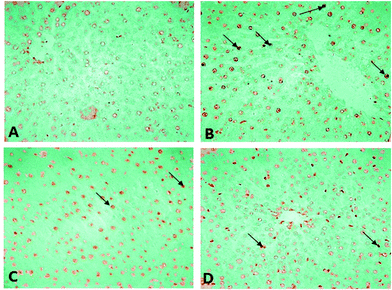
Histopathological changes in the liver of the arsenic and DDVP exposed rats (individually or in combination) are shown in Fig. 7. The severity of the lesions is also summarized in Table 1. The histology of the control rat liver showed normal morphology of the hepatic parenchyma, hepatic lobules and hepatocytes arranged in cords radiating from the central canal (Fig. 7). Liver sections of the arsenic alone exposed rat showed minimal to mild hepatocellular degeneration and vacuolation along with proliferation of bile ducts and hyperactivation of Kupffer cells. Multiple focal periportal and mid-zonal areas of necrosis along with infiltration of monocytoid cells were also observed. The liver of DDVP alone exposed rats showed mild to moderate distortion of normal hepatic morphology, hepatocytic vacuolation, pyknotic hepatocytes, and areas of sporadic necrosis surrounded by the infiltrating monocytoid cells. When animals were co-exposed to arsenic and DDVP, the liver histology showed greater severity of degenerative changes in the hepatic parenchyma as compared to the animals exposed to arsenic or DDVP alone. Rats with arsenic or DDVP exposure alone or in combination showed both oncotic (cellular swelling) and apoptotic types of cell injury; apoptotic cells were confirmed by the TUNEL assay (Table 3). The prevalence of TUNEL positive cells was higher in DDVP alone and in combination with arsenic and DDVP exposed animals. Control rats rarely showed TUNEL positive cells (Fig. 8 and 9).| S. no. | Lesions | Control | DDVP | As | DDVP + As |
|---|---|---|---|---|---|
| a n = 5. −, nil; +, minimal (<12%); ++, mild (<22%); and +++, moderate (<45%). | |||||
| 1 | Hepatocellular degeneration | − | +++ | + | +++ |
| 2 | Hemorrhage | − | + | − | − |
| 3 | Hypertrophy of Kupffer cells | − | ++ | + | + |
| 4 | Clumping of cytoplasm | − | + | ++ | + |
| 5 | Necrosis/apoptosis | + | +++ | ++ | +++ |
| 6 | Proliferation of bile duct parenchyma | − | − | − | + |
| 7 | Karyolysis/chromatolysis | − | ++ | + | ++ |
| 8 | Hepatocellular vacuolation | − | − | ++ | − |
| 9 | Polymorphonuclear cell infiltration | − | + | + | − |
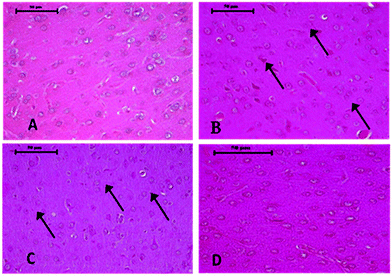
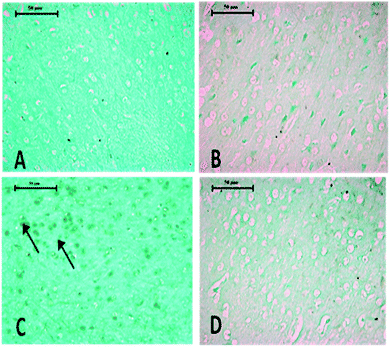
Histopathological changes in the brain following exposure to arsenic and DDVP individually or in combination are shown in Fig. 6. The control rat brain showed normal neurons, with glial cells arranged in several layers. Rats with arsenic exposure showed mild gliosis (proliferation of glial cells), shrunken pyknotic neurons along with central chromatolysis, edema and mild to moderate neuronal vacuolation. Animals with DDVP exposure showed mild to moderate chromatolysis, gliosis and edema along with necrotic and degenerative changes. Animals with arsenic and DDVP co-exposure showed mild to moderate gliosis, however other degenerative lesions were less pronounced compared to arsenic or DDVP exposure alone as shown in Tables 1 and 2. In this study TUNEL positive cells were only observed in arsenic exposed rat brain as shown in Table 3.
| S. no. | Lesions | Control | DDVP | As | DDVP + As |
|---|---|---|---|---|---|
| a n = 5. −, nil; +, minimal (<12%); and ++, mild (<22%). | |||||
| 1 | Neuronal degeneration | − | + | + | − |
| 2 | Neuronal vacuolation | − | − | − | − |
| 3 | Neurophagia | − | − | − | − |
| 4 | Necrosis | − | ++ | + | ++ |
| 5 | Engorged blood vessels | − | ++ | + | + |
| 6 | Gliosis | − | + | ++ | + |
| 7 | Neuronal basophilia | − | + | ++ | ++ |
| 8 | Pyknosis | − | − | − | − |
| 9 | Edema | − | + | ++ | − |
| S. no. | Lesions | Control | DDVP | As | DDVP + As |
|---|---|---|---|---|---|
| a n = 5. −, nil; +, minimal (<12%); ++, mild (<22%); and +++, moderate (<45%). | |||||
| 1 | TUNEL apoptotic cells in brain | − | − | ++ | − |
| 2 | TUNEL apoptotic cells in liver | − | +++ | ++ | +++ |
Discussion
Exposure of humans and animals to arsenic and organophosphate pesticides is currently one of the most serious global health concerns especially in developing countries. Enough literature is available for the individual toxic effects of these toxicants, but very few studies have been done to investigate metal–pesticide interactions. The few isolated reports which are available suggest the mechanism for these interactions to be associated with alterations in hepatic cytochrome P450-dependent mono-oxygenase activity.32 We earlier provided some interesting observations for the possible interaction between organophosphate pesticides and arsenic suggesting that their concomitant exposure does not necessarily lead to synergistic effects.33 Thus, the current study was planned to further explore these interactions and also to delineate the possible mechanism for the observed effects.We observed that during co-exposure to arsenic and DDVP, brain arsenic concentration was marginally less compared to their individual exposure. Similarly liver arsenic concentration too showed a marginal but non-significant increase in animals co-exposed to arsenic and DDVP compared to arsenic alone. However these changes were only marginal and statistically non-significant and thus not much emphasis should be given to these moderate variations. However, we observed a much more pronounced difference in these results in a few earlier studies reported from our laboratory utilizing different doses and duration of exposure.33 We attributed these alterations to the possible formation of an arsenic–OP complex leading to the accumulation of arsenic in liver in groups receiving arsenic with DDVP. This complex may affect the absorption and distribution of these toxicants in the body. We also suggested formation of a possible arsenic–OP complex involves the release of arsenic from the liver due to DDVP competition for complexation with arsenic.33
There have been various reports confirming the entry of arsenic into the brain.34 The present study confirms the access of arsenic to the brain tissue. Organophosphate pesticides, being neurotoxic in nature, are known to cross the blood–brain barrier and alter the brain biogenic amine level.20,35,36 In the present experiment, a significant decrease in neurotransmitter level in arsenic and DDVP exposed animals has been observed, suggesting a possible mechanism associated with the delayed neurotoxicity of OP. The decreased levels of these (NE and DA) neurotransmitters may be due to the decreased activity of tyrosine hydroxylase (TH; a rate limiting enzyme in catecholamine synthesis) and dopamine-β-hydroxylase enzymes involved in the synthesis of NE and DA. The 5-HT level remained unaltered on exposure to arsenic and DDVP.34,37 A moderate insignificant increase in AChE activity in the arsenic alone exposed group was observed, which is in agreement with our previous report where the change was more significant compared to the increase reported in this study.33,38 This increase may be attributed to the interaction of metals with acetylcholine receptors affecting their binding efficiency resulting in increased AChE synthesis.38 DDVP could not produce AChE inhibition in the presence of arsenic suggesting that DDVP and arsenic may mask the toxic effect of each other on AChE activity when they exist concomitantly. Meanwhile the DDVP alone exposed group significantly inhibited AChE activity which is a well known target for OP toxicity. These altered biogenic amines suggested possible involvement of free radicals resulting in oxidative stress. In order to demarcate the role of oxidative stress in neurotoxicity, hepatotoxicity and cell death, various biochemical parameters in both brain and liver were investigated. These biochemical variables were further supported by histopathological observations indicating disturbed pro-oxidant/antioxidant balance and ultimately leading to neural and hepatic injury.
Oxidative stress is reported as a major underlying mechanism of OP and arsenic toxicity.14,39 The effects after co-exposure to these toxicants on oxidative stress were evaluated in the current study. Oxidative stress results from the generation of increased free radicals and parallel depletion of antioxidant enzymes causing oxidation of various macromolecules such as DNA, protein and lipids. The antioxidant defense system prevents oxidative stress by scavenging free radicals like ROS. Glutathione serves as a second line of defense in the body and plays an important role in preventing free radicals with its –SH group. This –SH group becomes oxidized to form a disulfide bond known as GSSG.
In the present study, we observed significant alterations in ROS and GSH levels in animals exposed to arsenic and DDVP respectively. The lowered glutathione level appears to be the first indicator of oxidative stress, although TBARS (a marker of lipid peroxidation) was significantly elevated in all the individual groups in the brain and for DDVP in the liver. Interestingly, the co-exposed groups showed varied results and exhibited only a moderate non-significant increase in the case of ROS while antagonism was observed in TBARS. No change in liver GSH and GSSG levels was noted. These interesting observations may be due to the complex formation between these toxicants.33 This hypothesis was supported in our earlier published studies where an elevated liver arsenic concentration was noted but no such significant elevation of liver-As concentration was observed in the present study as the increase was only marginal. These are important observations and do require further exploration. ROS can also react with phospholipid moieties of the cellular membrane and these moieties reside within polyunsaturated fatty acids at high concentrations. ROS identify methylene groups present in phospholipids and oxidizes them for further ROS production. Lipid peroxidation acts as one of the mechanisms by which ROS cause damage to cell membranes and intracellular cytoplasmic organelles.21,40
Glutathione related enzymes like GPx, GST and GR function in the detoxification process to regulate cellular homeostasis. Our results showed that there was a significant alteration in the GSH and GSSG levels. For recycling of these enzymes, GPx and GR play an important role because they catalyzes the conversion of GSH into GSSG and regeneration of GSH from GSSG. GPx also converts lipid peroxides along with hydrogen peroxides therefore we examined the rate of lipid peroxidation.41 Activities of GPx and GR are strictly regulated with the changes in GSH level.
In the current study, the increase in GPx activity was accompanied by a decrease in the activity of GR and decreased levels of GSH. These observations indicate that GPx efficiently converted GSH to GSSG and the decreased GR activity explains the fact that GSH content was not rapidly recovered. It can thus be hypothesized that altered glutathione linked enzymes lead to an oxidative imbalance and result in increased cell death. On the other hand, the significant elevation of GST activity observed in the present study suggests a protective response to eliminate toxicants.34,40,42 During co-exposure to arsenic and DDVP, we observed only a moderate decrease in GSH levels accompanied by a small increase in GSSG, ROS, GST and GPx activity compared to the control group. These are interesting observations and suggest that combined exposures to these toxicants may lead to more pronounced toxicity. However, as the changes described above were only marginal, no definite conclusion can be drawn and perhaps a more exhaustive study addressing these issues may be required to arrive at a definite conclusion.
Accumulative reactive oxygen species (ROS) production and depletion of antioxidant enzymes play a critical role in causing cell death and apoptosis. Our results suggest that chronic arsenic or DDVP exposure induces hepatic and neuronal cell death by increasing oxidative stress. In the present study, histopathological examination clearly supported the biochemical observation with respect to arsenic concentration in soft tissues. Histopathological examinations of the liver showed mild hepatocellular degeneration, necrosis/apoptosis with hypertrophy of Kupffer cells, and karyolysis in the arsenic exposed group, while the DDVP exposed group showed mild to moderate signs of clumping of cytoplasm and vacuolation along with necrosis/apoptosis. The combined effects of arsenic or DDVP were marginally elevated as compared to individual arsenic or DDVP exposure.43,44 Histology of the brain showed mild gliosis, neuronal basophilia along with neuronal degeneration and necrosis in the arsenic and DDVP alone exposed groups, while concomitant exposure to arsenic and DDVP also caused cellular damage. These effects were more pronounced as compared to the individual exposure. These observations conflict with the previous findings.37,45 However, there is no available report on co-exposure to arsenic and OP suggesting a possible mechanism supported by histopathological examinations. Further study is needed to explore the possible mechanism.
Arsenic or DDVP induced cell death in the histopathological observations was further investigated by performing TUNEL staining in tissues to ascertain apoptotic changes. Apoptosis is an active mode of cell death frequently referred to as “programmed cell death” in which unneeded or diseased cells die, while necrosis is an uncontrolled cell death. In this study, histopathological observations showed cells with apoptotic features as well as with necrotic features and to confirm the specific cell death, the deoxyribonucleotidyl transferase (TDT)-mediated dUTP-digooxigenin nick-end labeling (TUNEL) assay was carried out for further verification. Brain tissues showed an increase in the TUNEL positive cells in the arsenic exposed animals, while increased TUNEL positive cells were observed in the liver of the DDVP alone and co-exposed groups. However, a prominent prevalence of TUNEL positive cells was seen in the liver.37,45–47 The study suggests that (i) individual exposure to arsenic or DDVP induces neuronal damage mediated by alterations in biogenic amines and hepatotoxicity by excessive generation of ROS; (ii) concomitant exposure to arsenic and DDVP results in synergistic effects accompanied with some antagonistic effects.
Abbreviations
| DDVP | Dichlorvos |
| As | Arsenic |
| GSH | Glutathione |
| GSSG | Oxidized glutathione |
| ROS | Reactive oxygen species |
| TBARS | Thiobarbituric acid reactive substances |
| SOD | Superoxide dismutase |
| CAT | Catalase |
| GPx | Glutathione peroxidase |
| GST | Glutathione-S-transferase |
| NE | Norepinephrine |
| DA | Dopamine |
| 5-HT | 5-Hydroxytryptamine |
Conflict of interest
The authors declares that there are no conflict of interest.Acknowledgements
Authors thank the Director, Defence Research and Development Establishment for his support and encouragement.References
- S. J. S. Flora, Free Radicals Biol. Med., 2011, 51, 257–281 CrossRef CAS PubMed.
- K. Jomova, Z. Jenisova, M. Feszterova, S. Baros, J. Liska, D. Hudecova, C. J. Rhodes and M. Valko, J. Appl. Toxicol., 2011, 31, 95–107 CAS.
- T. Roychowdhury, Int. J. Hyg. Environ. Health, 2010, 213, 414–427 CrossRef CAS PubMed.
- WHO, Guidelines for drinking water quality, World Health Organization, Geneva, 3rd edn, 2004 Search PubMed.
- B. K. Mandal, T. R. Chowdhury, G. Samanta, G. K. Basu, P. P. Chowdhury, C. C. Chanda, D. Lodh, N. K. Karan, R. K. Dhar, D. K. Tamili, D. Das, K. C. Saha and D. Chakraborti, Curr. Sci., 1996, 70, 976–986 CAS.
- B. K. Mandal and K. T. Suzuki, Talanta, 2002, 58, 201–235 CrossRef CAS.
- S. Nain and J. E. Smits, Environ. Toxicol., 2012, 27, 244–254 CrossRef CAS PubMed.
- S. M. Nagvi, C. Vaishnavi and H. Singh, in Arsenic in the environment. Part II: Human Health and Ecosystem Effects, ed. J. E. Nriagu, John Wiley and Sons, Inc, New York, 1994, pp. 55–91 Search PubMed.
- J. Ecobichon, Toxic effects of pesticides, in Casarett and Doull's Toxicology, ed. C. D. Klaassen, McGraw-Hill, 6th edn, 1996, pp. 763–810 Search PubMed.
- J. Storm, K. Rozman and K. Doull, Toxicology, 2000, 150, 1–29 CrossRef CAS.
- D. M. Quin, Chem. Rev., 1987, 87, 955–979 CrossRef.
- S. Xi, L. Guo, R. Qi, W. Sun, Y. Jin and G. Sun, J. Biochem. Mol. Toxicol., 2010, 24, 368–378 CrossRef CAS PubMed.
- S. Shila, V. Kokilavani, M. Subathra and C. Panneerselvam, Toxicology, 2005, 210, 25–36 CrossRef CAS PubMed.
- S. J. S. Flora, Clin. Exp. Pharmacol. Physiol., 1999, 26, 865–869 CrossRef CAS.
- R. Dringen, J. M. Gutterer and J. Hirrlinger, Eur. J. Biochem., 2000, 267, 4912–4916 CrossRef CAS.
- G. P. Das, G. P. Shaik and K. Jamil, Drug Chem. Toxicol., 2006, 29, 147–156 CrossRef CAS PubMed.
- A. M. Saleh, C. Vijayasarathy, M. Fernandez-Cabezudo, M. Taleb and G. Petroianu, J. Appl. Toxicol., 2003, 23, 23–29 CrossRef CAS PubMed.
- M. Abdollahi, A. Ranjbar, S. Shadnia, S. Nikfar and A. Rezaie, Med. Sci. Monit., 2004, 10, RA141–RA147 CAS.
- J. P. Groten, F. R. Cassee, P. J. Van Bladeren, C. DeRosa and V. J. Feron, Mixtures, in Toxicology, ed. H. Marguardt, S. G. Schafer, R. McClellan and F. Welsch, Academic Press, New York, 1999, pp. 257–270 Search PubMed.
- N. Dwivedi, Y. D. Bhutia, V. Kumar, P. Yadav, P. Kushwaha, H. Swarnkar and S. J. S. Flora, Human Exp. Toxicol., 2010, 29, 121–129 CAS.
- P. Kaur, B. Radotra, R. W. Minz and K. D. Gill, Neurotoxicology, 2007, 28, 1208–1219 CrossRef CAS PubMed.
- M. Modi, R. K. Kaul, G. M. Kannan and S. J. S. Flora, J. Trace Elem. Med. Biol., 2006, 20, 197–204 CAS.
- D. J. Socci, K. B. Bjugstad, H. C. Jones, J. V. Pattisapu and G. W. Arendash, Exp. Neurol., 1999, 155, 109–117 CrossRef CAS PubMed.
- H. Ohkawa, N. Ohishi and K. Yagi, Anal. Biochem., 1979, 93, 351–358 CrossRef.
- P. J. Hissin and R. Hilf, Anal. Biochem., 1976, 74, 214–226 CrossRef CAS.
- L. Flohe and W. A. Gunzler, Methods Enzymol., 1984, 105, 114–121 CrossRef CAS.
- W. H. Habig, M. J. Pabst and W. B. Jakohy, J. Biol. Chem., 1974, 249, 7130–7139 CAS.
- G. Saxena and S. J. S. Flora, J. Biochem. Mol. Toxicol., 2004, 18, 221–233 CrossRef CAS PubMed.
- G. L. Ellman, K. D. Courtney, V. Andres and R. M. Feaderstone, Biochem. Pharmacol., 1961, 7, 88–95 CrossRef CAS.
- D. M. Jacobwitz and J. S. Richardson, Pharmacol. Biochem. Behav., 1978, 8, 515–519 CrossRef.
- H. M. Stahr, Arsenic, in Analytical Toxicology Methods Manual, ed. H. M. Stahr, Iowa State University Press, Ames, Iowa, 1977, pp. 80–83 Search PubMed.
- J. K. Malik and A. G. Telang, in Pesticides: Metabolism, Neurotoxicity and Epidemiology, John Wiley and Sons, Inc, New York, 2010, pp. 329–339 Search PubMed.
- N. Dwivedi and S. J. S. Flora, Food Chem. Toxicol., 2011, 49, 1152–1159 CrossRef CAS PubMed.
- H. Soininen, L. Unni and S. Shillcutt, Neurochem. Res., 1990, 15, 1185–1190 CrossRef CAS.
- S. J. S. Flora, M. Mittal and D. Mishra, J. Neurol. Sci., 2009, 285, 198–205 CrossRef CAS PubMed.
- I. Celik, Z. Yilmaz and V. Turkoglu, Environ. Toxicol., 2009, 24, 128–132 CrossRef CAS PubMed.
- B. K. Binukumar and K. D. Gill, Indian J. Exp. Biol., 2010, 48, 697–709 CAS.
- A. C. D. Bainy, M. H. Gennari de Medeiros, P. D. Mascio and E. A. de Almeida, Rev. Biotemas, 2006, 19, 35–39 Search PubMed.
- S. P. Llopis, M. D. Ferrando and J. B. Pena, Aquat. Toxicol., 2003, 65, 337–360 CrossRef.
- I. Celik and H. Suzek, Ecotoxicol. Environ. Saf., 2009, 72, 905–908 CrossRef CAS PubMed.
- A. L. Hussain, Food Chem. Toxicol., 2006, 46, 82–86 CrossRef PubMed.
- D. Mishra, A. Mehta and S. J. S. Flora, Chem. Res. Toxicol., 2008, 21, 400–407 CrossRef CAS PubMed.
- S. K. Yaduvanshi, A. Ojha, S. C. Pant, V. Lomash and N. Srivastava, Pestic. Biochem. Physiol., 2010, 97, 214–222 CrossRef CAS PubMed.
- N. Benjamin, A. Kushwah, R. K. Sharma and A. K. Katiyar, Indian J. Exp. Biol., 2006, 44, 228–232 Search PubMed.
- S. J. S. Flora, M. Mittal, V. Pachauri and N. Dwivedi, Metallomics, 2012, 4, 78–90 RSC.
- A. Santra, A. Chowdhury, S. Ghatak, A. Biswas and G. Krishna Dhali, Toxicol. Appl. Pharmacol., 2007, 220, 146–155 CrossRef CAS PubMed.
- S. Bashir, Y. Sharma, M. Irshad, S. D. Gupta and T. D. Dogra, Basic Clin. Pharmacol. Toxicol., 2006, 98, 38–43 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |