Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Electrochemical synthesis of flat-[Ga13−xInx(μ3-OH)6(μ-OH)18(H2O)24(NO3)15] clusters as aqueous precursors for solution-processed semiconductors†
Matthew E.
Carnes
*,
Christopher C.
Knutson
,
Athavan
Nadarajah
,
Milton N.
Jackson
Jr.
,
Anna F.
Oliveri
,
Kevin M.
Norelli
,
Brandon M.
Crockett
,
Sage R.
Bauers
,
Hidekel A.
Moreno-Luna
,
Benjamen N.
Taber
,
Daniel. J.
Pacheco
,
Jarred Z.
Olson
,
Kaylena R.
Brevick
,
Claire E.
Sheehan
,
Darren W.
Johnson
and
Shannon W.
Boettcher
*
Department of Chemistry & Biochemistry, the Materials Science Institute, the Center for Sustainable Materials Chemistry, University of Oregon, Eugene, Oregon 97403-1253, United States. E-mail: mec2105@gmail.com; swb@uoregon.edu
First published on 29th August 2014
Abstract
Flat-[Ga13(μ3-OH)6(μ-OH)18(H2O)24](NO3)15 (Ga13) and heterometallic [Ga13−xInx(μ3-OH)6(μ-OH)18(H2O)24](NO3)15 (x = 5, 4) clusters were synthesized by the electrolysis of metal nitrate salt solutions to directly form, without purification, aqueous precursor inks for InxGa13−xOy semiconducting films in <2 h. Raman spectroscopy and 1H-NMR spectroscopy confirm the presence of [Ga13−xInx(μ3-OH)6(μ-OH)18(H2O)24(NO3)15] clusters. Bottom-gate thin-film transistors were fabricated using ∼15 nm-thick Ga13−xInxOy films as the active channel layer, displaying turn-on voltages of −2 V, and on/off current ratios greater than 106. The average channel mobility of the transistors fabricated from the cluster solutions generated by electrolysis was ∼5 cm−2 V−1s−1 which was more than twice that of transistors fabricated from control solutions with the simple nitrate salt precursors of ∼2 cm−2 V−1s−1. Electrochemical cluster synthesis thus provides a simple and direct route to aqueous precursors for solution-processed inorganic electronics.
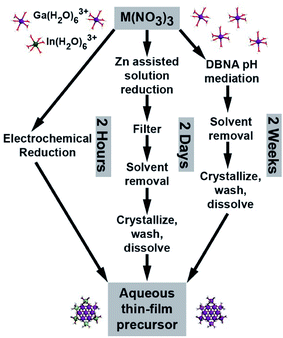
Thin film deposition using aqueous inorganic-cluster precursors provides an alternative to traditional vacuum processing techniques for thin-film deposition.1,2 As one example, “flat” Group 13 [M13(μ3-OH)6(μ-OH)18(H2O)24(NO3)15], homo- and heterometallic clusters (Fig. 1) have been used to deposit high-performance semiconductor3 and dielectric films.4 Because of this, significant effort has been aimed at improving Group-13 cluster synthesis. Early syntheses took two weeks and used dibutylnitrosamine (DBNA), a known carcinogen.3,5 Wang et al. showed that the addition of Zn powder to acidic Al(NO3)3 solutions results in condensation of [Al13(μ3-OH)6(μ-OH)18(H2O)24(NO3)15] (Al13) clusters via a gradual pH increase of the solution through nitrate reduction. The reaction is complete in approximately two days and the carcinogenic DBNA is no longer needed.6 A disadvantage to this method is that extensive purification is required to remove Zn2+ from the precursor solution. The preferential solubility of zinc nitrate in alcohol is used to purify the clusters, as M13 clusters are negligibly soluble in many organic solvents. In contrast, electrochemistry provides a direct mechanism to drive reduction reactions without the use of chemical reagents that must be later removed. Recently, both flat7 and Keggin8 Al13 clusters have been electrochemically synthesized.
Here we report the electrochemical synthesis of [Ga13−xInx(μ3-OH)6(μ-OH)18(H2O)24]15+ (x = 0, 4, 5) clusters and show that the aq. solutions obtained can be used, without purification, to deposit Ga13−xInxOy channel layers with good thin-film transistor (TFT) performance. The elimination of secondary reagents and purification steps is beneficial for mass production, sustainability, and cost. Films can be cast directly from the modified salt solutions, making this a direct method for obtaining various homo- and heterometallic Group 13 oxide thin films with a variety of applications.
The synthesis is performed in a two-compartment electrochemical cell comprised of a beaker housing (1) the Pt working electrode, a Ag/AgCl reference electrode, and a pH probe, and (2) a Pt counter electrode inside a medium-fritted glass tube that serves as a separate counter electrode compartment (Fig. S1†). Experimental details are provided in the ESI.† The applied working electrode potentials were chosen to be slightly negative of the reduction potential of the metal cations at the pH of interest as described by their Pourbaix diagrams.9 Potentials of −1.00 V vs. Ag/AgCl for Ga and −0.49 V vs. Ag/AgCl for Ga–In mixtures were used to generate the desired products with the given apparatus. The voltage of −1.00 V for aq. solutions of gallium nitrate caused a change in the luster of the Pt surface which could be seen by eye.10 Yields of washed product show this plating results in a relatively small amount of Ga loss overall (<2%).
The primary mechanism of this reaction appears to be the removal of nitrate from the solution via its reduction to ammonium, NOx, and other species. The removal of nitrate counter anions from the solution raises the pH of the solution by consuming protons as in, e.g., eqn (1) and thus drives the formation of the cluster via LeChatelier's principle as it acts on the reaction as given in eqn (2).
| NO3− + 2e− + 3H+ ⇒ HNO2 + H2O | (1) |
| 13M(H2O)6(NO3)3 ⇌ [M13(μ3-OH)6(μ-OH)18(H2O)24](NO3)15 + 30H2O + 24HNO3 | (2) |
Analysis of an air-dried aliquot of the crude reaction by 1H-NMR shows a prominent triplet peak with equal peak heights corresponding to the 1H–14N coupling of ammonium ions centered at 7.1 ppm (ref. 11) (Fig. S3†). This indicates that nitrate is reduced to ammonium as a part of one pathway in which counterions are removed from solution and the pH is raised. Although the presence of ammonium ions indicates that nitrate reduction is involved in raising the pH of the cluster solution and forcing olation of the metal aqua species, it does not rule out other contributing mechanisms. We find that electrolysis at sufficiently high current results in evolution of a brown gas. This is likely due to the reduction of NO3− to NOx gases.12 We performed the electrochemical synthesis of Ga13 and Ga13−xInx mixed clusters at a constant applied voltage which was high enough to reduce small amounts of metal but low enough to prevent large losses of material to plating. We believe that some metal plating onto the electrode is important to condition the Pt toward nitrate reduction. Nitrate can undergo a number of reduction processes to form species including N2O4, HNO2, NO, and NH4+. The standard reduction potentials are similar, between +0.8–1.0 V vs. NHE,13a and all much more positive than the hydrogen reduction potential. At a clean Pt electrode, however, H2 generation might be expected to dominate given the fast kinetics relative to nitrate reduction. We did not observe significant bubbles (that would be associated with H2 formation) on the Pt electrode surface. After Pt is modified by Ga–In plating it likely becomes poisoned for hydrogen evolution and thus kinetically favors the nitrate reduction reaction.13b These data support the hypothesis that nitrate reduction is the predominant electrochemical reaction. Regardless of the cathode reaction, charge balance requires additional positively charged species (e.g. In(H2O)63+ or Ga(H2O)63+) to migrate from the counter electrode compartment into the working electrode compartment or negatively charged species (e.g. NO3−) to migrate the opposite direction. Both migration processes serve to lower the nitrate-to-metal-ion ratio in the working-electrode-compartment film-precursor solution.
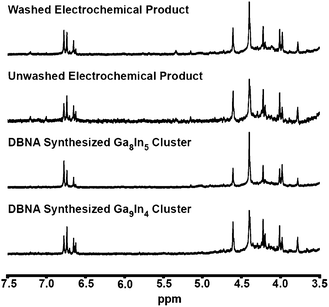
Proton NMR provides useful information for the identification and determination of the degree of substitution by indium in heterometallic clusters. Analysis of aq. inorganic clusters by 1H-NMR spectroscopy is traditionally challenging in protic solvents due to acidic proton exchange with the solvent. In most aprotic solvents, analysis of inorganic clusters by 1H-NMR spectroscopy is made difficult by the low solubility of highly-charged clusters. These obstacles are overcome by using d6-DMSO, which allows for the detection of signals arising from water molecules and hydroxide bridges of the cluster. To confirm the presence of clusters, a portion of the electrochemically generated samples was air dried and then dissolved in d6-DMSO. These samples were allowed to equilibrate overnight to ensure even DMSO exchange at the outer hydroxyl shell of the clusters.14 The 1H-NMR spectra of the reduced Ga(NO3)3 product is consistent with that of flat-Ga13 clusters previously reported (Fig. S3†).15
Using 1H-NMR, we are able to distinguish between differently substituted heterometallic clusters once they have been dried and isolated. After equilibrating in d6-DMSO for 24 h, the clusters for each Ga![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) In ratio gives rise to a distinctive spectrum with a clearly developed fingerprint region (ESI Fig. S4†). Although we can identify the Ga
In ratio gives rise to a distinctive spectrum with a clearly developed fingerprint region (ESI Fig. S4†). Although we can identify the Ga![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) In ratio from this signature, we are still unable to distinguish between positional isomers of the In at the exterior of the clusters. Crystals were grown of each of the isomers independently and their spectra taken to calibrate the results.16 The 1H-NMR spectra obtained for the product of the mixed metal nitrate reduction, starting with a 6
In ratio from this signature, we are still unable to distinguish between positional isomers of the In at the exterior of the clusters. Crystals were grown of each of the isomers independently and their spectra taken to calibrate the results.16 The 1H-NMR spectra obtained for the product of the mixed metal nitrate reduction, starting with a 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 ratio of Ga to In, is consistent with the Ga9In4 cluster synthesized independently (ESI Fig. S4†). After washing the product of the electrochemical reaction with isopropanol, this product appears to have exchanged some of the external metal ions to form Ga8In5 clusters as is evident by the change in their distinctive 1H-NMR spectra. This suggests that the clusters may be dynamic in the presence of the washing solvent and that In readily substitutes for Ga within the cluster (Fig. 2). Exchange of In atoms around flat M13 has recently been observed in solution to be a reversible, equilibrium process.16
7 ratio of Ga to In, is consistent with the Ga9In4 cluster synthesized independently (ESI Fig. S4†). After washing the product of the electrochemical reaction with isopropanol, this product appears to have exchanged some of the external metal ions to form Ga8In5 clusters as is evident by the change in their distinctive 1H-NMR spectra. This suggests that the clusters may be dynamic in the presence of the washing solvent and that In readily substitutes for Ga within the cluster (Fig. 2). Exchange of In atoms around flat M13 has recently been observed in solution to be a reversible, equilibrium process.16
We find evidence for M13 species forming with fewer reducing equivalents than that reported for the Zn-based synthesis of [Al13(μ3-OH)6(μ-OH)18(H2O)24(NO3)15].6a Ga13 clusters are observed after passing a cathodic charge of 0.7–0.8 electrons per Ga, and 0.4–0.5 electrons per metal in the case of the Ga13−xInx clusters. The Zn-based synthesis of Al13 used 1.0 reducing equivalents per Al (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 Zn
2 Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al as Zn is a 2e− reductant). The synthesis of a related Sc2 cluster used 0.75 reducing equivalents per Sc.6b Our hypothesis to explain such behavior is that if hydroxyl-bridged metal cluster formation is under equilibrium control, not all of the excess nitrate counterions need to be consumed for clusters to form. Our analysis does not however exclude the possibility that the reaction does not go to completion under the conditions used. Nitrate ions can also be effectively removed from association with the growing clusters by counterbalancing the positive charge associated with newly formed ammonium ions, leaving this new ammonium nitrate salt in solution but allowing ions to diffuse away from clustering species.
Al as Zn is a 2e− reductant). The synthesis of a related Sc2 cluster used 0.75 reducing equivalents per Sc.6b Our hypothesis to explain such behavior is that if hydroxyl-bridged metal cluster formation is under equilibrium control, not all of the excess nitrate counterions need to be consumed for clusters to form. Our analysis does not however exclude the possibility that the reaction does not go to completion under the conditions used. Nitrate ions can also be effectively removed from association with the growing clusters by counterbalancing the positive charge associated with newly formed ammonium ions, leaving this new ammonium nitrate salt in solution but allowing ions to diffuse away from clustering species.
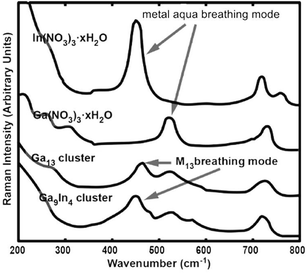
Raman spectroscopy is also useful for identifying M13 clusters.17 The Raman spectra of aliquots from the electrochemical synthesis agree with previous reports of Ga13 clusters, highlighted by the ν1 Ga–O symmetric stretch, or breathing mode at 464 ± 1 cm−1 (Fig. 3).17 The Raman spectra of the structurally analogous Ga13−xInx cluster reveal similar vibrational features to those observed in Ga13 clusters, with the ν1 breathing mode slightly red-shifted to 449 ± 1 cm−1. This shift is consistent with the substitution of the larger In for Ga, and with the observed difference between the vibrational modes of In and Ga hexa-aqua salts (Fig. 3).
The class of flat M13 Group 13 clusters prepared previously have been shown to be effective precursors for high-quality thin films.3,4 In this study, aq. cluster-containing solutions with an In![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga ratio of 6
Ga ratio of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
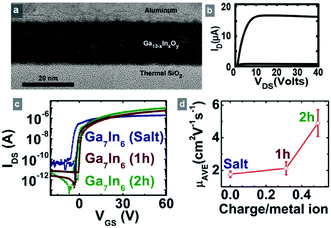
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 produced by the electrochemical synthesis were directly spin-cast onto thermally grown SiO2 on Si wafers and annealed at 550 °C. This process circumvents the recrystallization step and the need to wash and dissolve the solid products in another solvent, thus reducing the time and solvent needed for synthesis. Heterometallic clusters were used to generate channel layers within TFTs. A TEM image of a device cross-section confirms the uniform morphology of thin films processed from the electrochemically-synthesized precursor (Fig. 4a). EDX measurements of the films (Fig. S5†) confirmed the presence of both In and Ga in the films.
7 produced by the electrochemical synthesis were directly spin-cast onto thermally grown SiO2 on Si wafers and annealed at 550 °C. This process circumvents the recrystallization step and the need to wash and dissolve the solid products in another solvent, thus reducing the time and solvent needed for synthesis. Heterometallic clusters were used to generate channel layers within TFTs. A TEM image of a device cross-section confirms the uniform morphology of thin films processed from the electrochemically-synthesized precursor (Fig. 4a). EDX measurements of the films (Fig. S5†) confirmed the presence of both In and Ga in the films.
Fig. 4b–d show the device properties of the heterometallic cluster channel layer in TFTs processed from the electrochemically generated cluster solutions and compares them to those made using a starting nitrate salt solution. The devices derived from electrochemically-synthesized precursors are comparable to previously reported devices using DBNA-derived precursors.3 Devices obtained from cluster precursors show on-to-off current ratios of greater than 106 and turn-on voltages near −2 V whereas the devices made from starting salt solution show slightly negative turn-on voltages near −3 V and on-to-off ratios greater than 105 (Fig. 4c).
The average channel mobility of cluster films are greater than those obtained from starting salt solution films by at least a factor of two (Fig. 4d). These values for mobility were calculated by the method of Wager et al. and should be considered the average mobility of the accumulated charge in the channel.18 Compared to the mixed salt solutions of In(NO3)3 and Ga(NO3)3, the Ga13−xInx clusters have fewer nitrate counter ions per active metal because the nitrates are consumed electrochemically during the cluster synthesis. This decrease in nitrate concentration drives olation and preorganization of the metal hydroxides into clusters.19 Because nitrates must be removed during the annealing step to give an oxide thin film, we attribute the enhanced performance of the electrolyzed solution to reduced porosity in the final semiconductor channel that would be caused by decomposing counter ions.
Although the goal of this work is to show the new electrochemical synthesis route yields cluster precursors whose TFT performance is similar to clusters made by conventional methods, it is also useful to compare the performance to other solution-derived oxide thin films. Kim et al. reported the use of “combustion processing” to deposit related In–Zn–O films at temperatures as low as 200 °C from methoxyethanol solutions.20a Composition-optimized In0.7Zn0.3O1.35 devices fabricated with a SiO2 gate dielectric (as is done here) had saturation mobilities (μsat) of 10 cm2 V−1 s−1 after annealing at 400 °C. Hwang et al. reported μsat of 8 cm2 V−1 s−1 for In0.7Zn0.3O1.35 after annealing at 300 °C when Zn(NO3)2 and In(NO3)3 were deposited from an aqueous solution.20b The In0.46Ga0.53O1.5 studied here had average channel mobilities of 5 cm2 V−1 s−1. Studies of vapor-deposited films show that mobility increases sharply with higher In concentration.20c Increasing the In![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga ratio in the clusters would be expected to further increase TFT performance. Alternative gate dielectrics (e.g. amorphous alumina20a), and surface/interface passivation layers,21 also dramatically improve the TFT performance of films made from other solution precursors. These strategies can directly be used to improve the performance of the cluster precursors reported here.
Ga ratio in the clusters would be expected to further increase TFT performance. Alternative gate dielectrics (e.g. amorphous alumina20a), and surface/interface passivation layers,21 also dramatically improve the TFT performance of films made from other solution precursors. These strategies can directly be used to improve the performance of the cluster precursors reported here.
In summary, an alternate synthetic method is reported for the synthesis of flat homo- and heterometallic Group 13 cluster precursor solutions that can be directly used in the fabrication of thin-film transistors. This new method reduces the processing time to generate M13 cluster solutions from two days to two hours. The synthesis is carried out electrochemically so as to reduce protons and nitrate ions in a controlled fashion. Heterometallic clusters synthesized using this method are functionally similar in transistor applications to previously synthesized and characterized clusters.3 These films are capable of being spin-cast directly from unpurified reaction solutions into high-quality thin films. The films are dense, smooth, and processable at relatively moderate temperatures under ambient atmospheric conditions. This reagent-free, electrochemical synthesis may also find application in future mechanistic studies of cluster formation and speciation.
Acknowledgements
This work was funded by National Science Foundation (NSF) grant CHE-1102637 and was the result of a research-based immersion course in cluster/film chemistry developed by the NSF Center for Sustainable Materials Chemistry. We acknowledge Jeffrey Ditto and Josh Razink for assistance in electron beam imaging. The CAMCOR shared instrument facilities are supported by grants from the W.M Keck Foundation, the M.J. Murdock Charitable Trust, ONAMI, the Air Force Research Laboratory (FA8650-05-1-5041), NSF (0923577 and 0421086) and the University of Oregon. SWB acknowledges support from the Research Corporation for Science Advancement as a Cottrell Scholar.Notes and references
- (a) K. Jiang, A. Zakutayev, J. Stowers, M. D. Anderson, J. Tate, D. H. McIntyre, D. C. Johnson and D. A. Keszler, Solid State Sci., 2009, 11, 1692–1699 CrossRef CAS; (b) K. Jiang, J. T. Anderson, K. Hoshino, D. Li, J. F. Wager and D. A. Keszler, Chem. Mater., 2011, 23, 945–952 CrossRef CAS; (c) S. T. Meyers, J. T. Anderson, D. Hong, C. M. Hung, J. F. Wager and D. A. Keszler, Chem. Mater., 2007, 19, 4023–4029 CrossRef CAS; (d) S. T. Meyers, J. T. Anderson, C. M. Hung, J. Thompson, J. F. Wager and D. A. Keszler, J. Am. Chem. Soc., 2008, 130, 17603–17609 CrossRef CAS PubMed; (e) J. T. Anderson, C. L. Munsee, C. M. Hung, T. M. Phung, G. S. Herman, D. C. Johnson, J. F. Wager and D. A. Keszler, Adv. Funct. Mater., 2007, 17, 2117–2124 CrossRef CAS; (f) M. Alemayehu, J. E. Davis, M. Jackson, B. Lessig, L. Smith, J. D. Sumega, C. Knutson, M. Beekman, D. C. Johnson and D. A. Keszler, Solid State Sci., 2011, 13, 2037–2040 CrossRef CAS; (g) A. Nadarajah, M. E. Carnes, M. G. Kast, D. W. Johnson and S. W. Boettcher, Chem. Mater., 2013, 25, 4080 CrossRef CAS.
- (a) R. Murugavel, M. G. Walawalkar, M. Dan, H. W. Roesky and C. N. R. Rao, Acc. Chem. Res., 2004, 37, 763–774 CrossRef CAS PubMed; (b) K. L. Fujdala and T. D. Tilley, J. Am. Chem. Soc., 2001, 123, 10133–10134 CrossRef CAS PubMed; (c) P. Sekar, E. C. Greyson, J. E. Barton and T. W. Odom, J. Am. Chem. Soc., 2005, 127, 2054–2055 CrossRef CAS PubMed; (d) H. S. Kim, P. D. Byrne, A. Facchetti and T. J. Marks, J. Am. Chem. Soc., 2008, 130, 12580–12581 CrossRef CAS PubMed.
- Z. L. Mensinger, J. T. Gatlin, S. T. Meyers, L. N. Zakharov, D. A. Keszler and D. W. Johnson, Angew. Chem., Int. Ed., 2008, 47, 9484–9486 CrossRef CAS PubMed.
- S. W. Smith, W. Wang, D. A. Keszler and J. F. Conley Jr, J. Vac. Sci. Technol., A, 2014, 32, 041501 Search PubMed.
- (a) J. T. Gatlin, Z. L. Mensinger, L. N. Zakharov, D. Macinnes and D. W. Johnson, Inorg. Chem., 2008, 47, 1267–1269 CrossRef CAS PubMed; (b) Z. L. Mensinger, W. Wang, D. A. Keszler and D. W. Johnson, Chem. Soc. Rev., 2012, 41, 1019–1030 RSC.
- (a) W. Wang, K. M. Wentz, S. E. Hayes, D. W. Johnson and D. A. Keszler, Inorg. Chem., 2011, 50, 4683–4685 CrossRef CAS PubMed; (b) W. Wang, I. Y. Chang, L. Zakharov, P. H.-Y. Cheong and D. A. Keszler, Inorg. Chem., 2013, 52, 1807–1811 CrossRef CAS PubMed.
- W. Wang, W. Liu, I.-Y. Chang, L. A. Wills, L. N. Zakharov, S. W. Boettcher, P. H.-Y. Cheong, C. Fang and D. A. Keszler, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 18397 CrossRef CAS PubMed.
- C. Hu, H. Liu and J. Qu, Colloids Surf., A, 2005, 260, 109–117 CrossRef CAS.
- M. Pourbaix, Atlas of electrochemical equilibria in aqueous solutions, National Association of Corrosion Engineers, 2nd English edn, Houston, 1974 Search PubMed.
- W. M. Saltman and N. H. Nachtrieb, J. Electrochem. Soc., 1953, 100, 126–130 CrossRef CAS.
- E. Pretsch, P. Bühlmann and M. Badertscher, Structure Determination of Organic Compounds: Tables of Spectral Data, 4th edn, Springer-Verlag, Berlin, 2009 Search PubMed.
- M. C. P. M. da Cunha, J. P. I. De Souza and F. C. Nart, Langmuir, 2000, 16, 771–777 CrossRef CAS.
- (a) Standard Potentials in Aqueous Solutions, A. J. Bard, R. Parsons and J. Jordan, ed. International Union of Pure and Applied Chemistry, 1985 Search PubMed; (b) T. Mahle, Metallic and bimetallic catalysts for electrochemical reduction of problematic aqueous anions, Masters Thesis, University of Illinois, Urbana-Champagne, 2012 Search PubMed.
- Samples are prepared for NMR by dissolving the sample in d6-DMSO and allowing them to set for 24 h. Before that time the spectra is too complicated to analyze. Slow exchange with the solvent with the external waters of the cluster eventually results in a more simplified spectrum with a distinct fingerprint region. Fig. S4 in the ESI† shows how this fingerprint region changes as a function of In substitution within crystallographically confirmed Ga13−xInx clusters.
- A. F. Oliveri, M. E. Carnes, M. M. Baseman, E. K. Richman, J. E. Hutchison and D. W. Johnson, Angew. Chem., Int. Ed., 2012, 51, 10992–10996 CrossRef CAS PubMed.
- M. K. Kamunde-Devonish, M. N. Jackson, Jr, Z. L. Mensinger, L. N. Zakharov and D. W. Johnson, Inorg. Chem., 2014, 53(14), 7101–7105 CrossRef CAS PubMed.
- M. N. Jackson, Jr, L. A. Wills, I.-Y. Chang, M. E. Carnes, L. Scatena, P. H.-Y. Cheong and D. W. Johnson, Inorg. Chem., 2013, 52, 6187–6192 CrossRef PubMed.
- D. Hong, G. Yerubandi, H. Q. Chiang, M. C. Spiegelberg and J. F. Wager, Crit. Rev. Solid State Mater. Sci., 2008, 33, 101–132 CrossRef CAS.
- M. Henry; J. P. Jolivet and J. Livage, Aqueous Chemistry of Metal Cations: Hydrolysis, Condensation and Complexation, Structure and Bonding, Springer-Verlag, Berlin, 1992, vol. 77 Search PubMed.
- (a) M.-G. Kim, M. G. Kanatzidis, A. Facchetti and T. J. Marks, Nat. Mater., 2011, 10, 382 CrossRef CAS PubMed; (b) Y. H. Hwang, J.-S. Seo, J. M. Yun, H. Park, S. Yang, S.-H. K. Park and B.-S. Bae, NPG Asia Mater., 2013, 5, e45 CrossRef CAS; (c) T. Kamiya and H. Hosono, NPG Asia Mater., 2010, 2, 15 CrossRef.
- (a) Y. S. Rim, H. Chen, X. Kou, H.-S. Duan, H. Zhou, M. Cai, H. J. Kim and Y. Yang, Adv. Mater., 2014, 26, 4273 CrossRef CAS PubMed; (b) R. A. Street, T. N. Ng, R. A. Lujan, I. Son, M. Smith, S. Kim, T. Lee, Y. Moon and S. Cho, ACS Appl. Mater. Interfaces, 2014, 6, 4428 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, NMR and EDX spectra. See DOI: 10.1039/c4tc01354a |
| This journal is © The Royal Society of Chemistry 2014 |