Thermal degradation of the green-emitting SrSi2O2N2:Eu2+ phosphor for solid state lighting
Abstract
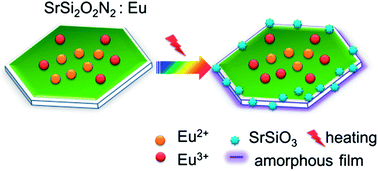
A phase pure SrSi2O2N2:Eu2+ green phosphor was synthesized by a solid state reaction through careful control of the Sr : Si ratio in the starting powder consisting of SrCO3, Si3N4 and Eu2O3. The thermal degradation of the phosphor was investigated by baking it at high temperatures for 2 h. The surface states of the samples before and after baking were analyzed by SEM, HRTEM, XPS, TGA/DTA, and high temperature in situ X-ray diffraction. The results showed that the thermal degradation became intense when the temperature was higher than 500 °C, and the degradation was caused by the formation of SrSiO3 on the particle surface and the oxidation of Eu2+ to Eu3+. It is suggested that the thermal stability can be enhanced by achieving high crystallinity as well as high phase purity of the phosphor.

 Please wait while we load your content...
Please wait while we load your content...