Twisted intramolecular charge transfer, aggregation-induced emission, supramolecular self-assembly and the optical waveguide of barbituric acid-functionalized tetraphenylethene†
Abstract
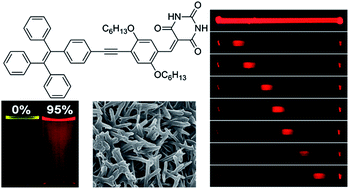
A red-emissive barbituric acid-functionalized tetraphenylethene derivative (TPE-HPh-Bar) was designed and synthesized. TPE-HPh-Bar exhibits the effect of twisted intramolecular charge transfer due to the interaction of its donor and acceptor units. Whereas TPE-HPh-Bar emits faintly in solution, it becomes a strong emitter in the aggregated state, demonstrating a phenomenon of aggregation-induced emission. TPE-HPh-Bar can self-assemble into nanospheres upon natural evaporation of its solutions. In the presence of melamine, nanorods and (un)sealed nanotubes are formed, the content of which depends on the melamine amount. The crystalline nanorods of TPE-HPh-Bar grown from diethyl ether/hexane solution exhibit a good optical waveguiding effect with a low optical loss (0.137 dB μm−1). Such attributes make the material to find wide applications in many areas such as biological imaging and optoelectronic nano-devices.
- This article is part of the themed collection: 2014 Journal of Materials Chemistry C Hot Articles

 Please wait while we load your content...
Please wait while we load your content...