 Open Access Article
Open Access ArticleFunctionalized carbon nanotubes as transporters for antisense oligodeoxynucleotides†
Anika
Kaufmann
*a,
David
Kunhardt
b,
Giuseppe
Cirillo
bc,
Silke
Hampel
b and
Bernd
Schwenzer
a
aChair of Biochemistry, Department of Chemistry, Technische Universität Dresden, Bergstraße 66, D-01069 Dresden, Germany. E-mail: Anika.Kaufmann@chemie.tu-dresden.de
bLeibniz-Institut for Solid State and Materials Research Dresden, Helmholtzstraße 20, D-01069 Dresden, Germany
cDepartment of Pharmacy, Health and Nutritional Sciences, University of Calabria, Edificio Polifunzionale, I-87036 Rende (CS), Italy
First published on 18th August 2014
Abstract
The use of DNA-based therapeutics requires efficient delivery systems to transport the DNA to their place of action within the cell. To accomplish this, we investigated multiwalled carbon nanotubes (pristine MWCNT, p-MWCNT) functionalized with hydroxyl groups via 1,3-dipolar cycloaddition. In this way, we have obtained MWCNT-f-OH with improved stability in aqueous dispersions which is an advantageous property for their use in cellular environments. Afterwards, a carrier strand oligodeoxynucleotide (CS-ODN) was adsorbed to MWCNT-f-OH followed by hybridization with a therapeutic antisense oligodeoxynucleotide (AS-ODN). The amount of adsorbed CS-ODN, as well as the complementary AS-ODN and a non-complementary oligodeoxynucleotide (NS-ODN) as reference, was directly measured by radionuclide labeling of ODNs. We show that subsequent release of AS-ODNs and NS-ODNs was possible for MWCNT-f-OH above the melting temperature of AS-ODNs at 80 °C and under physiological conditions at different pH values at 37 °C. We also show a very low influence of p-MWCNT and MWCNT-f-OH on the cell viability of the bladder carcinoma (BCa) cell line EJ28 and that both MWCNT types were internalized by EJ28. Therefore, MWCNT-f-OH represents a promising carrier able to transport and release AS-ODNs inside cells.
Introduction
Carbon nanotubes (CNTs) are highly attractive as nanocarriers for biomedical applications.1–3 Due to their ability to penetrate cells they are able to transport therapeutics to their place of action.4,5 Therefore, they are interesting for novel DNA based therapeutics which must be transported efficiently into the cell. Additionally, CNTs can protect DNA against enzymatic cleavage and interference from nucleic acid binding proteins.6 So far, some studies have demonstrated the possibility of delivering plasmid DNA4 and siRNA7 by carbon nanotubes. In these studies DNA forms supramolecular complexes with functionalized single-walled CNTs (SWCNTs) through ionic interactions. Another possibility for DNA-based therapy is represented by antisense oligodeoxynucleotides (AS-ODNs) which are complementary to the mRNA of a selected gene and can therefore specifically inhibit the expression of that gene.8 The use of functionalized multiwalled CNTs (MWCNTs) for the intracellular delivery of quantum dot tagged AS-ODNs has been successfully shown by Jia et al.9 Since AS-ODNs are single-stranded oligodeoxynucleotides they could also be transported by a carrier strand oligodeoxynucleotide (CS-ODN). This CS-ODN can be either covalently or non-covalently coupled to CNTs whereas AS-ODNs can reversibly interact with the CS-ODN-modified CNTs by hybridization. In this way, AS-ODNs can be easily released after their transport into the cell.Whether hybridization occurs on the carbon nanotube surface is controversially discussed. Some groups found out that complementary ODN strands are displaced from carbon nanotubes after hybridization.10–12 Yang et al. used fluorescence anisotropy to confirm that hybridization occurs in solution rather than on the carbon nanotube surface.10 Additionally, dialysis was used to isolate dsDNA from the carbon nanotubes and the dialysis product with free dsDNA was analyzed by fluorescence spectroscopy10,11 and agarose gelelectrophoresis.12 On the other hand, Jeng et al. detected DNA hybridization on the surface of SWCNTs optically by using the near-infrared band-gap fluorescence of SWCNTs13,14 and Forster resonance energy transfer of fluorophore-labeled DNA oligonucleotides.14 DNA hybridization was also investigated successfully by UV-Vis-NIR absorption.15 Since carbon nanotubes act as quenchers for fluorophores,10,13,16 it is not possible to detect a fluorescent-labeled ODN strand directly on the carbon nanotube surface. This phenomenon is considered to base upon both energy-transfer and electron-transfer processes.10 Instead of fluorescence-labeling, we use radionuclide-labeled ODNs to detect the ODN strands directly on the carbon nanotube surface. In this way, we obtained for the first time a direct and quantitative proof for adsorption and hybridization of ODNs on the surface of MWCNTs.
For the use of CNTs as a delivery system, a main problem is their poor ability to form stable dispersions in aqueous suspensions, which is a key requirement for medical applications. Different strategies have been proposed to overcome this problem by functionalization with hydrophilic groups17,18 and noncovalent wrapping of DNA as some of the most significative approaches.19 Here, we modify MWCNTs with hydroxyl groups to increase their dispersion properties. Afterwards, we demonstrate that AS-ODNs could be released after hybridization to a CS-ODN adsorbed to these modified MWCNTs, offering a novel method to deliver AS-ODNs.
Experimental
Materials
ODNs with sequences according to Table 1 were purchased from biomers.net GmbH (Ulm, Germany). Radioisotopic labeling of ODNs was performed by Hartmann Analytics (Braunschweig, Germany). Centrifugal filters with modified nylon and a pore size of 0.2 μm for eppendorf tubes and KBr were obtained from VWR International GmbH (Darmstadt, Germany). 3,4-Dihydroxybenzaldehyde (DHBA), N,N-dimethylformamide (DMF), methanol and chloroform were purchased from Merck (Darmstadt, Germany). N-(Tri(hydroxymethyl)methyl)glycin (tricine) was obtained from Alfa Aesar (Karlsruhe, Germany). i-Propanol was purchased from Promochem (Wesel, Germany). 2-(4-(2-Hydroxyethyl)-1-piperazinyl)-ethansulfonsäure (HEPES) and 2-(N-morpholino) ethansulfonsäure (MES) were obtained from Sigma-Aldrich (Taufkirchen, Germany). Tris(hydroxymethyl)-aminomethane (Tris) was purchased from AppliChem GmbH (Darmstadt, Germany). Phosphate-buffered saline (PBS) with pH 7.4 was prepared by dissolving 4.5 g NaCl (VWR, Germany), 0.12 g KH2PO4 and 0.45 g Na2HPO4·2 H2O (Gruessing, Germany) in 500 mL distilled water and the pH was adjusted with NaOH (VWR, Germany).| Type | Sequence |
|---|---|
| CS-ODN | 5′-ACG CTG CCG CCA CCA CAC CA-3′ |
| AS-ODN | 5′-TGG TGT GGT GGC GGC AGC GT-3′ |
| NS-ODN | 5′-CCA AAC CCG TCA ATC AAG TC-3′ |
Preparation of p-MWCNT and MWCNT-f-OH
p-MWCNTs were synthesized by the fixed-bed chemical vapor deposition method as reported by Ritschel et al.20 The catalyst particles were removed by washing with diluted HNO3, which also leads to oxidation at the ends of p-MWCNTs.MWCNT-f-OH were synthesized via 1,3-dipolar cycloaddition.17,18 Briefly, 50 mg p-MWCNT were dispersed in 50 mL DMF for 30 min in an ultrasonic bath. Thereafter, 89.6 mg of the amino acid tricine and 103.6 mg DHBA were added and dispersed for additional 15 min in an ultrasonic bath. The dispersion was stirred for 96 h at 130 °C. After 24 h, 48 h and 72 h the same amounts of tricine and DHBA were again added. After the stirring process, the dispersion was filtered and washed with DMF, methanol and chloroform and dried overnight at 108 °C.
p-MWCNT: Raman ν (cm−1): 1350 (sp3 C); 1600 (sp2 C). FT-IR (KBr disk), ν (cm−1): 3435 (s, O–H); 1633 (s, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1384 (s, C–O).
O); 1384 (s, C–O).
MWCNT-f-OH: FT-IR (KBr disk), ν (cm−1): 3435 (s, O–H); 1633 (s, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O carboxyl); 1600 (s, aromatic C
O carboxyl); 1600 (s, aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 1450 (sc, CH2); 1384 (s, C–O carboxyl); 1200 cm−1 (s, C–O alcohol; s, C–N tricine).
C); 1450 (sc, CH2); 1384 (s, C–O carboxyl); 1200 cm−1 (s, C–O alcohol; s, C–N tricine).
Characterization of p-MWCNT and MWCNT-f-OH
The Raman experiments were performed using a Raman-Fourier-Transform-Spectrometer DXR SmartRaman (Thermo Fisher Scientific, USA) at a wavelength of λ = 532 nm and a laser power of 8 mW. The resolution of the spectrometer is 1 cm−1. FTIR spectra were recorded at room temperature in transmission mode with an IFS 113v spectrometer (Bruker, Germany) with a resolution of 0.5 cm−1. Thermogravimetric analysis was carried out in a SDT Q600 (TA Instruments, Germany) with a heating rate of 10 K min−1 and 100 mL min−1 synthetic air flow. The X-ray Photoelectron Spectroscopy (XPS) experiments were carried out in an ultrahigh vacuum system equipped with a hemispherical electron analyzer PHOIBOS 100 (SPECS, Germany) operating at a constant pass energy of 15 eV. The photoelectrons were excited with non-monochromatic Mg Kα (1253.6 eV) radiation. The X-ray source was run at a power of 300 W. The powder materials were fixed in special sample holders with moulds of 4 mm diameter; the analysis region is approx. 1 mm in diameter. The resolution is 0.2 eV. For the SEM analysis the CNT were individualized by sonication in i-propanol for 30 min. The resulting dispersion was dropped onto a 200 mesh coated copper grid with carbon-formvar and analyzed in a NanoSEM (FEI Company, Hillsboro, USA) using 15 kV acceleration voltage. TEM images were recorded in a Tecnai F30 (FEI Company, Hillsboro, USA) with 300 kV acceleration voltage.Adsorption of CS-ODNs to MWCNTs
In separate experiments, 1.0 mg mL−1 p-MWCNT and MWCNT-f-OH dispersions were prepared by dissolving p-MWCNT and MWCNT-f-OH in PBS for 30 min in an ultrasonic bath. Afterwards, 50 μL of this dispersion was mixed with 1 μM CS-ODN solution to a total volume of 500 μL PBS. The mixture of p-MWCNT or MWCNT-f-OH and CS-ODN was shaken for 30 min on a horizontal shaker at room temperature. Thereafter, p-MWCNT and MWCNT-f-OH were purified from free CS-ODN with a centrifugal filter by centrifugation for 3 min at 2300 rcf. For further purification two washing steps each with 500 μL PBS and subsequent centrifugation were performed.Hybridization and release of AS-ODNs and NS-ODNs
AS-ODNs or NS-ODNs were diluted to 0.5 μM in 200 μL PBS. The ODN solution was given to CS-ODNs adsorbed to p-MWCNT or MWCNT-f-OH directly on the filter membrane of centrifugal filters. After the hybridization time of 30 min at room temperature the solution was centrifuged through the centrifugal filter again to remove free AS-ODNs and NS-ODNs, respectively. Two subsequent washing steps each with 500 μL PBS and following centrifugation were performed for further purification. For release experiments, the appropriate buffer solution, PBS, MES (pH 5.2 and pH 6.0, 10 mM, 154 mM NaCl) and HEPES (pH 7.4 and pH 8.1, 10 mM, 154 mM NaCl), was given to the AS-ODN or NS-ODN hybridized CS-ODNs on p-MWCNT or MWCNT-f-OH on the centrifugal filter. The release was carried out at 80 °C whereas for temperature setting the Eppendorf thermal shaker 5436 was used. Afterwards, the solution was centrifuged through the centrifugal filter again to remove the released AS-ODNs and NS-ODNs, respectively.Radionuclide measurements
ODNs were labeled with 32P at the 3′-end and used at maximum twice the half-life counted from the reference date stated by the manufacturer. Adsorption, hybridization and release experiments were performed as described above except that the appropriate ODN was mixed with 32P-labeled ODN in fractions ranging from 0.01% to 0.5%, depending on the elapsed time from the reference date to maintain a sufficient count rate. A passivated implanted planar silicon detector based spectrometer system (Canberra/Ortec, Meriden, USA/Oak Ridge, USA) was used to determine the amount of 32P-labeled ODNs on the samples. The measured count rate was corrected by the background count rate measured with an empty centrifugal filter.Fluorescence spectroscopy measurements
For fluorescence spectroscopy AS-ODNs and NS-ODNs were labeled with fluorescein at the 5′-end. Hybridization of labeled AS-ODNs or NS-ODNs to CS-ODNs on p-MWCNT or MWCNT-f-OH was carried out as described above. Afterwards, the supernatant with released AS-ODNs and NS-ODNs could directly be measured. The measurements were performed with an F-4500 FL spectrophotometer from Hitachi (Tokyo, Japan) with time scan mode for 60 s. The excitation and emission wavelengths for fluorescein were λ = 494 nm and λ = 515 nm, respectively.Cell culture and cellular viability
The bladder carcinoma (BCa) cell line EJ28 (University of Frankfurt, Frankfurt, Germany) was cultured in Dulbecco's modified eagle's medium (DMEM, 4.5 g L−1 glucose) with 10% fetal calf serum, 1% gentamicin and 1% non-essential amino acids (all from GE Healthcare, Muenchen, Germany) in a humidified atmosphere containing 5% CO2 at 37 °C. Cellular viability was examined in quadruplicate after cell treatment using the cell proliferation reagent WST-1 (Roche, Mannheim, Germany).Cells treatment with MWCNTs and AS-ODNs
For MWCNT treatment, 300 cells per well were seeded in 96- well plates (Techno Plastic Products AG, Trasadingen, Switzerland) and allowed to adhere for 72 h. Cells were treated with different concentrations of CNT dispersions in DMEM. After 24 h the medium was changed and the cellular viability was measured 96 h after starting the treatment. For AS-ODN transfection and the combined treatment of AS-ODNs and MWCNT-f-OH 1500 cells per well were seeded in 96-well plates and allowed to adhere for 72 h. Afterwards, cells were transfected with 0.5 μM AS-ODNs using the liposomal transfection reagent N-[1-(2,3-dioleoyloxy)propyl]-N,N,N-trimethylammonium methyl-sulfate (DOTAP, Roche, Mannheim, Germany) (ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 w/w) in Opti-MEM (Life technologies GmbH, Darmstadt, Germany) which is a reduced serum medium for cationic lipid transfections. For the combined treatment AS-ODNs were mixed with MWCNT-f-OH in PBS and given to the cells in DMEM to a final concentration of 0.5 μM AS-ODNs and 0.1 mg mL−1 MWCNT-OH. After 4 h transfection time the medium was changed to DMEM and the cellular viability was measured 72 h after starting the treatment.
3 w/w) in Opti-MEM (Life technologies GmbH, Darmstadt, Germany) which is a reduced serum medium for cationic lipid transfections. For the combined treatment AS-ODNs were mixed with MWCNT-f-OH in PBS and given to the cells in DMEM to a final concentration of 0.5 μM AS-ODNs and 0.1 mg mL−1 MWCNT-OH. After 4 h transfection time the medium was changed to DMEM and the cellular viability was measured 72 h after starting the treatment.
Vascular endothelial growth factor (VEGF)-specific ELISA
The concentration of VEGF in culture medium was measured using a DuoSet ELISA Development System from R&D Systems (Wiesbaden, Germany) according to the manufacturers' instructions. The ELISA kit has been shown to recognize VEGF165, VEGF165b and VEGF121. As substrate solution the TMB Microwell Peroxidase Substrate System from Kirkegaard & Perry Laboratories, Inc (Gaithersburg, Maryland, USA) was used.Cytosol extraction
Cytosol extraction was modified based on the study by Staufenbiel and Deppert.21 EJ28 cells were cultured in 75 cm2 cell culture flasks (Techno Plastic Products AG, Trasadingen, Switzerland) to a growth density of 90–100%, washed with PBS and gathered in 1 mL 0.5 M Tris-HCl buffer (pH 7.4) with a cell scraper (Techno Plastic Products AG, Trasadingen, Switzerland). The cell suspension was centrifuged at 8000 rcf at 4 °C for 1 min. The cell pellet was resuspended in 150 μL 0.5 M Tris–HCl buffer (pH 7.4). The suspension was alternately incubated six times for 15 min at −80 °C and 5 min at 37 °C. Finally, the suspension was centrifuged at 8000 rcf at 4 °C for 10 min and the cytosol was gained in the supernatant. The protein content was determined using a bicinchoninic acid (BCA) protein assay kit from Thermo Scientific (Rockford, IL, USA).Bright field and fluorescence microscopy
The cells were transfected with fluorescein-labeled AS-ODNs as described above. Cells alone and cells treated with AS-ODNs without any transfection reagent served as control. 24 h after treatment the medium was changed against PBS and the samples were examined by bright field and fluorescence microscopy using an Axiovert 40 CFL microscope (Zeiss, Jena, Germany). Images were captured with an AxioCam MRm 5 camera and processed using Zen 2012 digital image processing software (both from Zeiss).TEM microscopy of MWCNT uptake into cells
The cellular uptake of p-MWCNT and MWCNT-f-OH was investigated by TEM studies with the cell line EJ28. 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells were seeded in 25 cm2 flasks and after 72 h adherent cells were treated with 0.1 mg mL−1 p-MWCNT or MWCNT-f-OH. Cells without MWCNT treatment served as control. After 24 h incubation cells were harvested by trypsin/EDTA treatment. The cell pellets were fixed in 3% glutaraldehyde, dehydrated in ethanol and embedded in epoxy resin. Ultrathin sections of the samples were cut on an ultramicrotome Ultracut R (Leica, Wetzlar, Germany) with a diamond knife, put onto 200 mesh coated nickel grids with formvar-carbon (Plano GmbH, Wetzlar, Germany) and examined with an EM900 transmission electron microscope (Zeiss, Jena, Germany).
000 cells were seeded in 25 cm2 flasks and after 72 h adherent cells were treated with 0.1 mg mL−1 p-MWCNT or MWCNT-f-OH. Cells without MWCNT treatment served as control. After 24 h incubation cells were harvested by trypsin/EDTA treatment. The cell pellets were fixed in 3% glutaraldehyde, dehydrated in ethanol and embedded in epoxy resin. Ultrathin sections of the samples were cut on an ultramicrotome Ultracut R (Leica, Wetzlar, Germany) with a diamond knife, put onto 200 mesh coated nickel grids with formvar-carbon (Plano GmbH, Wetzlar, Germany) and examined with an EM900 transmission electron microscope (Zeiss, Jena, Germany).
Statistical analyses
All radionuclide and fluorescence measurements were presented as mean (n = 3) ± standard deviation whereas cell culture measurements were presented as mean (n = 4) ± standard deviation.Results and discussion
Functionalization and characterization of MWCNTs
MWCNTs are carbon nanostructures easy to synthesize and handle, and thus were chosen as base materials for our study. We compared p-MWCNT and MWCNT-f-OH as potential nanocarriers for therapeutic AS-ODNs.p-MWCNT were synthesized by chemical vapor deposition and, as proven by a combined analysis of the SEM–TEM data, they consisted of individual filaments of graphene walls with average outer diameter and length of 11 and 980 nm, respectively (Fig. S1 and S2 in the ESI†). Through several washing steps carboxyl groups were introduced onto the surface of p-MWCNT. Afterwards, p-MWCNT were modified to develop hydrophilic properties on their surface by means of a 1,3-dipolar cycloaddition employing tricine and DHBA as reactants (Fig. 1A).
 | ||
| Fig. 1 (A) Reaction of p-MWCNT to MWCNT-f-OH; (B) dispersions of p-MWCNT (left) and MWCNT-f-OH (right) in PBS (1 mg mL−1). | ||
The p-MWCNT and the modified MWCNT-f-OH were dispersed in PBS to reach a concentration of 1 mg mL−1 (Fig. 1B). Thereby, MWCNT-f-OH showed better dispersion properties which were even stable for weeks, whereas for p-MWCNT, aggregates were clearly visible.
XPS confirmed the elemental distribution of the samples showing an increase of the nitrogen and oxygen contents of MWCNT-f-OH in comparison to p-MWCNT due to the covalent functionalization with tricine and DHBA (Table S1 in the ESI†). The reaction of p-MWCNT to MWCNT-f-OH was further characterized by Raman- and FT-IR spectroscopy as well as by thermogravimetric analyses (Fig. S3 in the ESI†). The Raman spectra (Fig. S3A†) showed that after functionalization, no change in the ID/IG value (and thus in the sp3/sp2 carbon ratio) occurred, indicating that through the cycloaddition reaction sp3 carbon as well as sp2 carbon was added and no further defects were formed. Comparing the FT-IR spectra of p-MWCNT and MWCNT-f-OH (Fig. S3B†) the effective functionalization of MWCNT-f-OH was confirmed by the remarkable increase in the band referable to the O–H, the presence of bands related to the DHBA and tricine residues.
The decomposition pattern of MWCNT-f-OH was similar but faster than that of p-MWCNT (thermogravimetric analyses, Fig. S3C†) due to the formation of pentagons containing nitrogen and the insertion of hydroxyl groups. This behavior, together with the absence of a degradation step at around T = 200 °C found in the physical mixture of DHBA, tricine, and MWCNT-f-OH, was used as confirmation of successful functionalization.
Adsorption of CS-ODNs to MWCNTs
For further applications in anticancer therapy, AS-ODNs should be transported by functionalized MWCNT-f-OH. A potential target for AS-ODN anticancer therapy is the expression of VEGF which plays an important role in the angiogenesis of cancer cells.22 We have chosen the AS-ODN sequence VEGF-723 which showed inhibition of the VEGF gene expression and reduced cell viability in the BCa cell line EJ28 and MCF-7 cells (breast carcinoma cell line).23First of all, we investigated the time and concentration dependency for the adsorption of CS-ODNs to MWCNT-f-OH (Fig. 2).
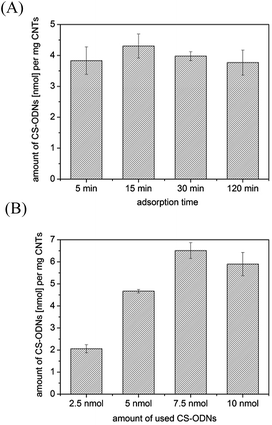 | ||
| Fig. 2 Adsorption of CS-ODNs to MWCNT-f-OH measured by radionuclide labeling; (A) time dependency of adsorption (c (CS-ODN) = 1 μM); (B) concentration dependency of CS-ODN-adsorption for t = 30 min. | ||
We found that adsorption of CS-ODN to MWCNT-f-OH was very fast and completed within minutes (Fig. 2A). A further increase of the adsorption time did not result in higher amounts of adsorbed CS-ODNs. The adsorption process showed a dependence on the CS-ODN concentration (Fig. 2B). An increase in the used amount of CS-ODNs caused a higher amount of adsorbed CS-ODNs on MWCNT-f-OH until the saturation was reached with 7.5 nmol of used CS-ODNs. The adsorption efficiency varied from 80% to 90% for 2.5 nmol, 5 nmol and 7.5 nmol of used CS-ODNs. After the saturation was reached the adsorption efficiency was reduced to 59% for 10 nmol of used CS-ODNs.
Hybridization of AS-ODNs to the carrier
In the next step, the hybridization of AS-ODNs to CS-ODNs adsorbed to MWCNT-f-OH was investigated. The hybridization of two complementary ODN strands in solution is very fast and normally completed within 1 min. Our results show that the hybridization of the AS-ODN to the CS-ODN adsorbed to MWCNT-f-OH was slower and reached a stable value not until 30 min (Fig. 3A). These data confirmed the results of Jeng et al. who demonstrated the hybridization kinetics of DNA to SWCNTs to be slower than hybridization in solution.13,14 Additionally, the hybridization was concentration dependent (Fig. 3B). For higher amounts of used AS-ODNs the amount of hybridized AS-ODNs to CS-ODN adsorbed to MWCNT-f-OH was increased. The hybridization efficiency varied from 30% for 1 and 10 nmol of used AS-ODNs to 40% for 2 and 4 nmol of used AS-ODNs.Releasing behavior of AS-ODNs from the carrier
For future applications in antisense therapy we investigated the possibility of releasing AS-ODNs from MWCNTs. Therefore, we compared the release of adsorbed AS-ODNs and AS-ODNs hybridized to CS-ODN-MWCNT-f-OH (Fig. 4).As a releasing condition we have chosen 80 °C, a value above the melting temperature of AS-ODN, to ensure that the hybridization of AS-ODNs with CS-ODN-MWCNT-f-OH is completely reversible. Obviously, only a small percentage of AS-ODNs, 0.15 nmol, was released when AS-ODNs were adsorbed to MWCNT-f-OH, whereas AS-ODNs hybridized to CS-ODN-MWCNT-f-OH were released more efficiently with an amount of 0.57 nmol. In this way, we proved that the hybridization with CS-ODNs is better suited to release AS-ODNs than the adsorption of AS-ODNs directly to MWCNT-f-OH. Furthermore, we investigated the specificity of AS-ODN hybridization to CS-ODN-MWCNT-f-OH in comparison to NS-ODNs. The amount of adsorbed NS-ODNs per mg CS-ODN-MWCNT-f-OH was as high as for AS-ODNs. Nevertheless, after 10 min in PBS at 80 °C, the amount of AS-ODNs was smaller than that of NS-ODNs. Therefore, the release of AS-ODNs was higher than for NS-ODNs due to the reversible hybridization to the CS-ODN. The difference between the release of AS-ODNs and NS-ODNs was not as high as expected. The main explanation is the unspecific adsorption of NS-ODNs to the MWCNT-f-OH surface which is not totally covered with CS-ODNs. The interaction of adsorbed NS-ODNs with MWCNT-f-OH was apparently higher than the hybridization interaction of AS-ODNs with ODN-MWCNT-f-OH. A possibility of reducing the unspecific binding of NS-ODNs to the surface of MWCNT-f-OH would be the use of longer CS-ODNs in order to achieve higher coverage of the MWCNT-f-OH surface with CS-ODNs which is planned in our ongoing work.
Furthermore, we investigated whether the modified MWCNT-f-OH are better suited for the release of AS-ODNs than the unmodified p-MWCNT. The amount of AS-ODNs and NS-ODNs per mg of p-MWCNT was twice as high as the amount of AS-ODNs and NS-ODNs per mg of MWCNT-f-OH, respectively (Fig. 5A). AS-ODNs hybridized to ODN-p-MWCNT were not released in PBS at 80 °C. In the case of p-MWCNT the π–π-stacking of nucleobases seems to be much higher and more stable as for MWCNT-f-OH. Therefore, AS-ODNs tend to adsorb to the surface of p-MWCNT rather than hybridize with the CS-ODN adsorbed to the surface. Due to their strong interaction with the surface of p-MWCNTs AS-ODNs and NS-ODNs were both not released. The releasing behavior for MWCNT-f-OH is much more desirable since very good first release of 70% AS-ODNs was reached. In comparison to AS-ODNs, only 50% of NS-ODNs were released from MWCNT-f-OH, which is direct proof for the specificity of hybridization. For MWCNT-f-OH hybridization of AS-ODNs to CS-ODNs seems to be more preferred and hence they can be reversibly released. NS-ODNs interact non-specifically with the CS-ODN and the surface of MWCNT-f-OH. But this interaction is not as high as with p-MWCNT. Therefore, MWCNT-f-OH are more promising for use as transporters in antisense therapy.
To prove our results using a second method, we also investigated an indirect method to measure the release of AS-ODNs and NS-ODNs based on fluorescence labeling. After the adsorption of CS-ODNs to MWCNTs, fluorescence labeled AS-ODNs could hybridize with the CS-ODNs adsorbed to p-MWCNTs and MWCNT-f-OH. Afterwards, AS-ODNs and NS-ODNs were released and separated by centrifugal filtration. In this way, p-MWCNTs and MWCNT-f-OH could not quench the fluorescence of labeled ODNs. To demonstrate the reproducibility of this method in comparison to radionuclide measurements, we repeated the experiment shown in Fig. 5A. Again, there was no release of AS-ODNs and NS-ODNs from p-MWCNT (Fig. 5B). For MWCNT-f-OH there was release of both AS-ODNs and NS-ODNs, with the release of AS-ODNs significantly higher due to specific hybridization. The release during the two washing steps was very low and can be neglected. During the radionuclide measurements the amount of released AS-ODNs during the first release was 0.58 nmol and 0.4 nmol for NS-ODNs whereas with fluorescence measurements 0.75 nmol of AS-ODNs and 0.3 nmol of NS-ODNs were released. Therefore, the values are quantitatively comparable within a certain error range due to two different measurement methods. The low amount of AS-ODNs and NS-ODNs in the supernatant of p-MWCNT samples indicates that AS-ODNs and NS-ODNs were adsorbed to p-MWCNT since hybridization is a reversible process which was demonstrated for MWCNT-f-OH. This result also indicates the reproducibility of the fluorescence and radionuclide measurements. Hence, fluorescence measurement provides supplementary results and represents an adequate alternative easier to handle and less risky for the investigator.
Since it is still under discussion whether hybridization occurs on the carbon nanotube surface13–15 or whether the carbon nanotube is displaced once the DNA strands hybridize,10–12 we would like to argue our point of view. With radionuclide labeling and quantification we have demonstrated that hybridization occurred directly on the CS-ODNs adsorbed to p-MWCNT and MWCNT-f-OH by measurements of complementary AS-ODNs. If AS-ODNs were displaced from the MWCNT surface directly after hybridization they would have been released after centrifugal filtration. Only hybridized AS-ODNs retained on the filter whereas released AS-ODNs can be found in the filtrate. Since the radionuclide labeled AS-ODNs were still measureable on the filter after different releasing steps it indicates that they were hybridized with CS-ODNs adsorbed to p-MWCNT and MWCNT-f-OH.
Releasing behaviour of AS-ODNs under physiological conditions
For the use of the proposed system in future therapeutic applications we compared the release at 80 °C, which is above the melting temperature, to the physiological temperature at 37 °C (Fig. S4 in the ESI†). At 80 °C, the release was very high after 10 min whereas at 37 °C the release was much lower. Therefore, we increased the time periods for the next releasing experiment at different pH values from 10 min to 1 h and 24 h.The release of AS-ODNs from the CS-ODN adsorbed to MWCNT-f-OH was strongly pH-dependent (Fig. 6).
Since DNA hybridization was also dependent on the ionic strength of the buffer we used MES (pH 5.2 and pH 6.0) and HEPES (pH 7.4 and pH 8.1) buffer with the same ionic strength. With increased pH values the release was much higher than for low pH values. At pH 5, there was nearly no release of AS-ODN and NS-ODN, and only a slight release was recorded at pH 6. The amount of AS-ODNs coupled to the carrier is 84% of the initial amount after the fourth release, whereas this value was 95% for NS-ODN at pH 6. At pH 7.4, the release was again increased, and after the fourth release, 20% and 66% of AS-ODNs and NS-ODNs were found on the carrier, respectively. The highest release was found at pH 8.1 where only 12% of AS-ODNs and 51% of NS-ODNs were found to be on MWCNT-f-OH after the fourth release. Therefore, the pH-dependency followed the same tendency as for hybridization of DNA in solution, where the stability of hybridization declines from acidic to basic pH values. At pH 7.4 and pH 8.1, there was also very fast release of AS-ODNs after 1 h (about 40% at pH 7.4 and 57% at pH 8.1), which was again increased after the longer time period of 24 h. Additionally, the release was clearly higher for AS-ODNs than for NS-ODNs at pH 7.4 and pH 8.1, what was a good indication of AS-ODN hybridization to the CS-ODNs on the surface of MWCNT-f-OH, since the hybridization is reversible at these pH values. The NS-ODNs were only adsorbed to the surface and therefore the release was significant lower. The cytosol of normal cells, but also that of tumor cells, has a neutral pH.24 Therefore, we expected that the AS-ODNs can be released intracellularly. To confirm this, we initially harvested the EJ28 cells in water and determined a pH of 7.1. Afterwards, we prepared the cytosol extract and performed release with different amounts of AS-ODNs adsorbed to MWCNT-f-OH (Fig. S5 in the ESI†). With half of the used AS-ODNs being released after 1 h, the release can be considered very fast. Previously, we determined that in buffer solution the release of AS-ODNs was better after hybridization to CS-ODN-MWCNT-f-OH. In cytosol the release of AS-ODNs is also high if they were directly adsorbed to MWCNT-f-OH, and this is the first indication that AS-ODNs could be transported by MWCNT-f-OH into cells.
Cellular uptake of MWCNTs and AS-ODNs
The BCa cell line EJ28 was treated with p-MWCNT and MWCNT-f-OH concentrations ranging from 0.025 mg mL−1 to 0.2 mg mL−1 for 24 h, and the cell viability was measured 96 h after starting the treatment (Fig. S6 in the ESI†). For both types of MWCNTs, the cell viability ranged between 80% and 100% at all concentrations. Therefore, MWCNTs alone have no relevant impact on the cell viability, which enables them as potential carriers for therapeutic AS-ODNs.Investigations on the cellular uptake of MWCNTs by TEM indicate that both p-MWCNT and MWCNT-f-OH were internalized into the cytoplasmic vacuoles of EJ28 (Fig. 7). Therefore, the uptake of MWCNTs is independent of the functional group which is in agreement with literature findings.5,23 Since the MWCNTs were mostly found in vacuoles, we assume that they were engulfed into cellular vesicles by energy dependent endocytic pathways which is one of the possible uptake mechanisms for CNTs.25–27
Using fluorescence microscopy we could show that fluorescein-labeled AS-ODNs can be transported into EJ28 cells with MWCNT-f-OH as carriers (Fig. 8). Cells alone and cells treated with AS-ODNs without any carrier show no fluorescence (Fig. 8 (A) and (B)). As positive control the cells were treated with AS-ODNs which were transported by the liposomal transfection reagent DOTAP which is normally used for the transfection of cells with AS-ODNs. It could be seen that some of the cells showed green fluorescence indicating that the fluorescein-labeled AS-ODNs were taken up by the cells (Fig. 8 (C)). Additionally, we could show that AS-ODNs were taken up into the cells when MWCNT-f-OH was used as a carrier instead of DOTAP (Fig. 8 (D)).
During cellular viability experiments, the AS-ODN VEGF-723 showed no influence on EJ28 cells (Fig. S7 in the ESI†). Therefore, we tested two different AS-ODN sequences (Table S2 in the ESI†) which were already found to possess good anti-cancer activity.23 The cell viability was reduced to 90% for AS-ODN VEGF-857 and to 82% for AS-ODN VEGF-859. When MWCNT-f-OH were used instead of DOTAP as transporters for AS-ODNs, the cell viability was decreased to 71% for AS-ODN VEGF-859 whereas the cell viability was reduced to 92% with MWCNT-f-OH alone. Therefore, the reduction had to be induced by the AS-ODN VEGF-859. With a VEGF-specific ELISA, we also investigated the influence on the protein expression after treatment with AS-ODNs and DOTAP or MWCNT-f-OH as transporters (Fig. S8 in the ESI†). Again the AS-ODN VEGF-723 showed nearly no influence on the VEGF protein expression of EJ28 whereas AS-ODN VEGF-859 reduced the VEGF protein expression to 72% in comparison to untreated cells. When using MWCNT-f-OH as a transporter for AS-ODN VEGF-859, the amount the VEGF protein expression was reduced to 95%.
Conclusions
In the present study, we modified and characterized MWCNT-f-OH which showed better dispersion properties in aqueous suspension than p-MWCNTs. For MWCNT-f-OH, it was possible to release AS-ODNs hybridized to a CS-ODN, whereas it was poorly possible for p-MWCNTs. The main advantage of our study was the labeling of ODNs with the radionuclide 32P. Therefore, we were able to examine for the first time the amount of ODNs directly adsorbed onto the surface of both types of MWCNTs. In this way, we demonstrated that hybridization occurs on the surface of MWCNTs rather than in solution. We also showed that adsorption and hybridization take place under the same conditions but only hybridization of AS-ODNs to CS-ODNs adsorbed to MWCNT-f-OH was reversible in buffer solutions. Release was possible at 80 °C but also under physiological conditions at 37 °C at pH 7.4 and at pH 8.1. AS-ODNs could also be released in cytosol extracts after direct adsorption to the MWCNT-f-OH surface. Furthermore, both MWCNTs have only a small influence on the cell viability at different concentrations for the BCa cell line EJ28. We also show cellular internalization of p-MWCNT and MWCNT-f-OH by EJ28 as well as uptake of AS-ODNs by MWCNT-f-OH into cells. The influence on the cellular viability and on the VEGF protein expression was not as high as expected. But for different AS-ODN sequences targeting the VEGF protein expression we determined an effect on the cellular viability and a small influence on the VEGF protein expression. We expect a higher effect on the cellular viability and protein expression with a greater transfection efficiency of MWCNT-f-OH which will be optimized in our ongoing work.MWCNT-f-OH represents a promising carrier to transport AS-ODNs into the cell. Since the amount of hybridized AS-ODNs is concentration-dependent, it is also possible to regulate the amount of AS-ODNs transported by a CS-ODN adsorbed to MWCNT-f-OH, making it suitable for different applications.
Acknowledgements
This study was supported by the German Cancer Aid (grant number 109617). G.C. is grateful for the financial support of Regional Operative Program (ROP) Calabria ESF 2007/2013-IV Axis Human Capital – Operative Objective M2 – Action D.5. The authors would like to acknowledge the team of the Central Radionuclide Laboratory of the University of Technology in Dresden for providing the analysis facilities, Prof. G. Seifert and PD Dr S. Füssel for helpful discussions as well as K. Wruck for the synthesis of the p-MWCNT, A. Schubert for washing the p-MWCNT, Dr S. Oswald and S. Kaschube for performing the XPS experiments and B. Hamann for TEM analysis of the cellular uptake of MWCNTs.Notes and references
- S. Hampel, D. Kunze, D. Haase, K. Krämer, M. Rauschenbach, M. Ritschel, A. Leonhardt, J. Thomas, S. Oswald, V. Hoffmann and B. Büchner, Nanomedicine, 2008, 3, 175 CrossRef CAS PubMed.
- S. Prakash, M. Malhotra, W. Shao, C. Tomaro-Duchesneau and S. Abbasi, Adv. Drug Delivery Rev., 2011, 63, 1340 CrossRef CAS PubMed.
- S. Peretz and O. Regev, Curr. Opin. Colloid Interface Sci., 2012, 17, 360 CrossRef CAS PubMed.
- D. Pantarotto, R. Singh, D. McCarthy, M. Erhardt, J.-P. Briand, M. Prato, K. Kostarelos and A. Bianco, Angew. Chem., Int. Ed., 2004, 43, 5242 CrossRef CAS PubMed.
- K. Kostarelos, L. Lacerda, G. Pastorin, W. Wu, S. Wieckowski, J. Luangsivilay, S. Godefroy, D. Pantarotto, J.-P. Briand, S. Muller, M. Prato and A. Bianco, Nat. Nanotechnol., 2007, 2, 108 CrossRef CAS PubMed.
- Y. Wu, J. A. Phillips, H. Liu, R. Yang and W. Tan, ACS Nano, 2008, 2, 2023 CrossRef CAS PubMed.
- M. A. Herrero, F. M. Toma, K. T. Al-Jamal, K. Kostarelos, A. Bianco, T. Da Ros, F. Bano, L. Casalis, G. Scoles and M. Prato, J. Am. Chem. Soc., 2009, 131, 9843 CrossRef CAS PubMed.
- M. E. Gleave and B. P. Monia, Nat. Rev. Cancer, 2005, 5, 468 CrossRef CAS PubMed.
- N. Jia, Q. Lian, H. Shen, C. Wang, X. Li and Z. Yang, Nano Lett., 2007, 7, 2976 CrossRef CAS PubMed.
- R. Yang, J. Jin, Y. Chen, N. Shao, H. Kang, Z. Xiao, Z. Tang, Y. Wu, Z. Zhu and W. Tan, J. Am. Chem. Soc., 2008, 130, 8351 CrossRef CAS PubMed.
- Y. Liu, Y. Wang, J. Jin, H. Wang, R. Yang and W. Tan, Chem. Commun., 2009, 665 RSC.
- R. J. Chen and Y. Zhang, J. Phys. Chem. B, 2006, 110, 54 CrossRef CAS PubMed.
- E. S. Jeng, A. E. Moll, A. Roy, J. B. Gastala and M. S. Strano, Nano Lett., 2006, 6, 371 CrossRef CAS PubMed.
- E. S. Jeng, P. W. Barone, J. D. Nelson and M. S. Strano, Small, 2007, 3, 1602 CrossRef CAS PubMed.
- C. Cao, J. H. Kim, D. Yoon, E.-S. Hwang, Y.-J. Kim and S. Baik, Mater. Chem. Phys., 2008, 112, 738 CrossRef CAS PubMed.
- Z. Zhu, R. Yang, M. You, X. Zhang, Y. Wu and W. Tan, Anal. Bioanal. Chem., 2010, 396, 73 CrossRef CAS PubMed.
- V. Georgakilas, K. Kordatos, M. Prato, D. M. Guldi, M. Holzinger and A. Hirsch, J. Am. Chem. Soc., 2002, 124, 760 CrossRef CAS PubMed.
- V. Georgakilas, A. Bourlinos, D. Gournis, T. Tsoufis, C. Trapalis, A. Mateo-Alonso and M. Prato, J. Am. Chem. Soc., 2008, 130, 8733 CrossRef CAS PubMed.
- M. Zheng, A. Jagota, E. D. Semke, B. A. Diner, R. S. McLean, S. R. Lustig, R. E. Richardson and N. G. Tassi, Nat. Mater., 2003, 2, 338 CrossRef CAS PubMed.
- M. Ritschel, A. Leonhardt, D. Elefant, S. Oswald and B. Büchner, J. Phys. Chem. C, 2007, 111, 8414 CAS.
- M. Staufenbiel and W. Deppert, Cell, 1983, 33, 173 CrossRef CAS.
- N. Ferrara, Oncologist, 2004, 9, 2 CrossRef CAS PubMed.
- Y. Förster, A. Meye, S. Krause and B. Schwenzer, Cancer Lett., 2004, 212, 95 CrossRef PubMed.
- J. R. Griffiths, Br. J. Cancer, 1991, 64, 425–427 CrossRef CAS.
- Q. Mu, D. L. Broughton and B. Yan, Nano Lett., 2009, 9, 4370 CrossRef CAS PubMed.
- C. P. Firme and P. R. Bandaru, J. Nanomed. Nanotechnol., 2010, 6, 245 CrossRef CAS PubMed.
- S. Boncel, K. H. Müller, J. N. Skepper, K. Z. Walczak and K. K. K. Koziol, Biomaterials, 2011, 32, 7677 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: XPS elemental distribution (%) of p-MWCNT and MWCNT-f-OH; SEM and TEM images of p-MWNTs; Raman, FT-IR and TGA of p-MWCNT and MWCNT-f-OH; release of AS-ODNs and NS-ODNs at 80 °C and 37 °C; release of AS-ODNs at 37 °C in cytosol; cellular viability of EJ28 after treatment with p-MWCNT and MWCNT-f-OH; cellular viability and VEGF protein expression of EJ28 after treatment with AS-ODNs transported by MWCNT-OH. See DOI: 10.1039/c4tb00915k |
| This journal is © The Royal Society of Chemistry 2014 |






