A novel zwitterionic bioceramic with dual antibacterial capability†
Montserrat
Colilla
*abc,
Marina
Martínez-Carmona
abc,
Sandra
Sánchez-Salcedo
abc,
M. Luisa
Ruiz-González
cd,
José M.
González-Calbet
cd and
María
Vallet-Regí
*abc
aDepartamento de Química Inorgánica y Bioinorgánica, Facultad de Farmacia, Universidad Complutense de Madrid, Instituto de Investigación Sanitaria Hospital 12 de Octubre i + 12, Plaza Ramón y Cajal s/n, 28040 Madrid, Spain. E-mail: mcolilla@ucm.es; vallet@ucm.es
bCenter on Bioengineering, Biomaterials and Nanomedicine (CIBER-BBN), Spain
cCEI Campus Moncloa, UCM-UPM, Madrid, Spain
dDepartamento de Química Inorgánica, Facultad de Químicas, UCM, Spain
First published on 18th June 2014
Abstract
A novel zwitterionic SBA-15 type bioceramic with dual antibacterial capability has been synthesized. The co-condensation route has been employed to functionalize SBA-15 with primary and secondary amine groups. The resulting material exhibits textural and nanostructural properties comparable to those of pure silica SBA-15, as confirmed by XRD, HR-TEM and N2 adsorption porosimetry. The presence of –NH3⊕/–SiO⊖ and >NH2⊕/–SiO⊖zwitterionic pairs on the material surface is evidenced by FTIR and 1H → 13C CP/MAS solid state NMR. The homogeneous distribution of this zwitterionic pairs agrees with the results derived from STEM-EDS studies. ζ-Potential measurements indicate that the zwitterionic nature of this material is preserved at the physiological pH of 7.4. In vitro bacterial assays using S. aureus demonstrate that the zwitterionic material is capable of inhibiting 99.9% of the bacterial adhesion compared to pure silica SBA-15. Moreover, cephalexin loading and delivery assays indicate that the zwitterionic sample is capable of releasing antibiotic molecules over long time periods. This dual antibacterial capability, i.e. antibiofouling and bactericidal, opens up promising expectations for the treatment of bone implant infections.
Introduction
Despite significant recent biomedical and engineering progress, infection remains one of the most severe and devastating complications associated with the use of implants for the treatment of bone diseases and fractures.1–4 In fact, bone implants are ideal substrates for bacterial colonization, since it is well-known that microorganisms prefer to colonize a solid substrate than dwell in a planktonic state.5 Following initial colonization bacteria grow and form communities called biofilms, which critically compromise the functionality and performance of the implant itself. Once a mature bacterial biofilm has established, conventional medical therapies based on systemic administration of antibiotics are not efficient and implant removal often constitutes the only possibility of eradicating the infection. This causes high morbidity and patient suffering and also constitutes an enormous cost for the national health systems.2,3Currently, much research effort is being dedicated to designing advanced bioceramics that exhibit antibacterial surfaces, i.e. surfaces capable of reducing the extent of attachment and proliferation of bacteria.6 Antibacterial surfaces may repel or resist the initial attachment of bacteria by either exhibiting an antibiofouling effect or by inactivating any cells coming into contact with the surface, therefore exhibiting a bactericidal effect.6 Among the chemical strategies to design antibiofouling or bacterial antiadhesive surfaces, functionalization with hydrophilic,7 antimicrobial8 and zwitterionic polymers and their derivatives9,10 has been reported. Recently, zwitterionization of bioceramics has been reported as a promising strategy to develop antibiofouling surfaces.11–13 In addition, bactericidal surfaces have been prepared by using diverse approaches such as using coatings containing silver and silver nanoparticles,14 grafting of antimicrobial peptides15,16 and quaternary ammonium compounds17 or loading bioceramic matrices with antibiotics.18–24 An innovative strategy to produce bioceramics with bactericidal capability consisted of grafting aminosilane derivatives to the surface of mesoporous silica materials, which were subsequently reacted or “charged” with NO. The NO release from the resulting materials led to biofilm eradication meanwhile exhibiting low in vitro cytotoxicity.25,26
The current challenge is to design antibacterial surfaces capable of combining antibiofouling and bactericidal capabilities for the dual treatment of implant infections. However, only a few strategies have been reported to attain this goal, which are based on the combination of polymeric surfaces capable of switching between attacking (e.g. cationic) and defending (e.g. zwitterionic) forms.27,28
Herein, we report the design and synthesis of a new nanostructured zwitterionic SBA-15 type bioceramic with dual antibacterial capability for the treatment of bone implant infections. The antibiofouling capability comes from the intrinsic zwitterionic nature of the surface, whereas the bactericidal potential results from its capability to host antibiotic drugs into the mesopores. Zwitterionic SBA-15 has been prepared by using an alkoxysilane bearing primary and secondary amine groups (N-(2-aminoethyl)-3-aminopropyl-trimethoxysilane, DAMO) and following the co-condensation route. Co-condensation offers several advantages compared to post-grafting functionalization.29 Since DAMO is a direct component of the silica matrix, pore blocking is not a problem using the co-condensation method. Besides, co-condensation usually leads to functionalized matrices where the organic units are generally more homogeneously distributed than the materials synthesized by the post-grafting process. Finally, the number of SiOH groups available to form zwitterionic species with amino groups from DAMO is higher when using the co-condensation route. Post-grafting functionalization would involve spending a great amount of surface SiOH groups of SBA-15 in forming covalent linkages (Si–O–Si) with DAMO. This would originate positively charged surfaces at the physiological pH of 7.4, as has been reported elsewhere.30 The co-condensation route permitted incorporating –NH3⊕/–SiO⊖ and >NH2⊕/–SiO⊖zwitterionic pairs onto the material surface, as evidenced by Fourier transform infrared spectroscopy (FTIR) and 13C solid state nuclear magnetic resonance (NMR). A deep study by high resolution transmission electron microscopy (HR-TEM) and energy dispersive scanning and transmission electron spectroscopy (STEM) coupled to energy dispersive X-ray spectroscopy (EDS) allowed investigating the nanostructure of the resulting materials and the distribution of functional groups in mesoporous crystals. ζ-Potential measurements were performed to determine the surface charge properties of this material vs. pH and to study if its zwitterionic nature was preserved under physiological conditions (pH ∼ 7.4). The antibacterial adhesion capability of zwitterionic SBA-15 compared to pure silica SBA-15 was in vitro tested using S. aureus, a microorganism usually involved in bone implant infections. Moreover, in vitro loading and release assays using cephalexin, a broad spectrum antibiotic used to treat common infections, were carried out.
Materials and methods
Synthesis of SBA-15 type mesoporous materials
A functionalized SBA-15 type silica mesoporous material exhibiting both primary and secondary amine groups, denoted as SBA-Zwitter, was synthesized by the co-condensation route using N-(2-aminoethyl)-3-aminopropyl-trimethoxysilane (DAMO, 97% wt, ABCR). Briefly, 8.0 g of Pluronic® P123 block copolymer ((PEO20PPO70PEO20) kindly provided by BASF Co.) was added to a mixture of 276 mL of H2O and 20.6 mL of concentrated HCl (37% wt, Aldrich).31 The solution was moderately stirred for 24 h at 35 °C until total surfactant dissolution. Then, 15 mL of tetraethyl orthosilicate (TEOS, 98% wt, Aldrich) was added. After ca. 20 min white powder appeared. Then, 1.64 mL of DAMO previously dissolved in 8.2 mL of isopropanol was dropwise added to the suspension. Mixtures were kept under magnetic stirring at 35 °C for 24 h and then sealed in glass beakers and heated at 100 °C for further 24 h. Finally, the obtained products were filtered, washed with deionized water, and then dried at 60 °C for 24 h. Then, the surfactant was removed by extraction.32 For this purpose, 1 g of mesoporous material was soaked into 100 mL of an isooctane–ethanol (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) mixture (isooctane, ≥99.5% wt, Aldrich; ethanol, 95%, Panreac) and kept under magnetic stirring at room temperature for 48 h. Afterward, the sample was filtered and then subjected to a second extraction process by soaking powder into 120 mL of an acetone–water (1
2) mixture (isooctane, ≥99.5% wt, Aldrich; ethanol, 95%, Panreac) and kept under magnetic stirring at room temperature for 48 h. Afterward, the sample was filtered and then subjected to a second extraction process by soaking powder into 120 mL of an acetone–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture (acetone, QP, Panreac) for 24 h at room temperature under magnetic stirring. After the extraction processes samples were left to dry in a vacuum oven for 24 h to ensure total solvent removal. For comparison, pure silica SBA-15 was also synthesized. Table 1 displays the molar compositions of the precursor sols used for the synthesis of different mesoporous materials.
1) mixture (acetone, QP, Panreac) for 24 h at room temperature under magnetic stirring. After the extraction processes samples were left to dry in a vacuum oven for 24 h to ensure total solvent removal. For comparison, pure silica SBA-15 was also synthesized. Table 1 displays the molar compositions of the precursor sols used for the synthesis of different mesoporous materials.
| Sample | TEOS | DAMO | P123 | HCl | H2O |
|---|---|---|---|---|---|
| SBA-15 | 1 | 0 | 0.017 | 3.4 | 208 |
| SBA-Zwitter | 0.90 | 0.10 | 0.017 | 3.4 | 208 |
Characterization of materials
The structural characteristics of the resulting materials were determined by powdered X-ray diffraction (XRD) in a Philips X'Pert diffractometer (Eindhoven, The Netherlands) equipped with CuKα (40 kV, 20 mA) over the range from 0.6 to 8.0° with a step of 0.02° and a contact time of 5 s. Electron microscopy was carried out in a JEOL 3000FEG transmission electron microscope operating at 300 kV and fitted with a X-ray energy dispersive spectrometer (EDS) (Oxford Instruments) and a Scanning Transmission Electron Microscopy (STEM) system.The textural properties of samples were determined by N2 adsorption porosimetry. The N2 adsorption/desorption analyses were carried out at −196 °C on a Micromeritics ASAP2020 analyzer (Micromeritics Co., Norcross, USA). In all cases, 50–70 mg of material was degassed at 90 °C for 24 h under a vacuum lower than 0.3 kPa before the analysis. The surface area was determined using the Brunauer–Emmett–Teller (BET) method.33 The total pore volume was estimated from the amount adsorbed at a relative pressure of 0.97. The estimation of microporous and mesoporous fractions to the total pore volume was performed by the t-plot method.34 The average mesopore size (DP) was obtained from the maximum of the pore size distribution calculated from the adsorption branch of the isotherm using the BJH method.35 The wall thickness (twall) was calculated by means of the expression twall = a0 − DP, where a0 is the unit cell parameter calculated from the d10 value of XRD using the expression a0 = d10 × 2/√3.36 To assess the possible existence of micropores (pore diameter < 2 nm) in samples, the t-plot method was employed, which allowed the estimation of the microporous fraction contribution (VμP) to the total pore volume.
The existence of functional groups and their chemical nature were investigated by FTIR in a Thermo Nicolet Nexus spectrometer equipped with a Goldengate attenuated total reflectance (ATR) device (Thermo scientific, USA). Quantitative determination of amino groups in the SBA-Zwitter sample sourced from DAMO was carried out by CHNS elemental chemical analysis in a Perkin-Elmer 2400CHNS thermo analyzer. The amount of residual surfactant in samples was calculated from CHNS analytic results and thermal analyses (TG and DTA). Thermal analyses were carried out under a dynamic air atmosphere between 30 and 950 °C (flow rate of 50 mL min−1 with a heating rate of 10 °C min−1) using a Perkin-Elmer Diamond analyzer (Perkin-Elmer, USA).
To investigate the cross-linking degree and the amount of silanol groups present in the synthesized materials 29Si magic angle spinning (MAS) solid-state nuclear magnetic resonance (NMR) measurements were performed in a Bruker AV-400-WB spectrometer (Karlsruhe, Germany) operating at 79.49 MHz. Solid samples were placed in a 4 mm zirconia rotor and spun at 10 kHz. Chemical shifts (δ) of 29Si were externally referred to 3-trimethylsilyl-1-propanesulfonic acid sodium salt (DDS) at δ = 0.0 ppm. Time periods between successive accumulations were 5 ms and ca. 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 scans were collected. The population of silanol groups per mol of silica was calculated from the relative population of silanol and geminal species, and divided by the weight per mol of silica materials (eqn (1)).37 The weight is derived from the relative populations and effective molecular weights (EMW) of the silanol, geminal, and siloxane species. The effective molecular weight of each species (EMWQ) is defined as the sum of the molecular weight of the atoms contributing to each species with the oxygen atoms in the siloxane bridges (Si–O–Si) that connect the species counted by half. The equation is:
000 scans were collected. The population of silanol groups per mol of silica was calculated from the relative population of silanol and geminal species, and divided by the weight per mol of silica materials (eqn (1)).37 The weight is derived from the relative populations and effective molecular weights (EMW) of the silanol, geminal, and siloxane species. The effective molecular weight of each species (EMWQ) is defined as the sum of the molecular weight of the atoms contributing to each species with the oxygen atoms in the siloxane bridges (Si–O–Si) that connect the species counted by half. The equation is:
 | (1) |
1H → 13C CP (cross-polarization)/MAS solid-state NMR measurements were performed to evaluate the different carbon environments in the synthesized samples. The spectrometer frequency was set to 75.45 MHz and samples were spun at 12 kHz. Chemical shift values were referenced to glycine. The time period between successive accumulations was 3 ms and the number of scans was ca. 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000.
000.
To evaluate the behavior of materials, regarding their surface charge properties in aqueous media, zeta-potential (ζ) measurements at different pH values were performed on a Malvern Zetasizer Nano Series instrument coupled to a MPT-2 multipurpose titrator from Malvern Instruments Ltd (UK). For this purpose, 5 mg of each powdered sample mesoporous was added to 5 mL of KCl 10 mM (used as the supporting electrolyte) and the pH was adjusted by adding appropriate volumes of 0.10 M HCl or 0.10 M KOH solutions.
In vitro bacterial adhesion assays
Bacterial adhesion assays on SBA-Zwitter and SBA-15 using Gram positive Staphylococcus aureus (American Type Culture Collection, ATCC 28213) were performed under static conditions, as commonly reported in the literature.12,38–41To perform the bacterial adhesion experiments on the different powdered materials, disk-shaped pieces of 6 mm diameter and 1 mm height were prepared by compacting fractions of 20 mg of dried powders using 2.75 MPa uniaxial pressure. Prior to adhesion assay, samples were sterilized by UV irradiation for 7 min on each side of the piece.
S. aureus were grown to a mid-logarithmic phase in Todd Hewitt broth (THB) medium (Sigma-Aldrich, USA) at 37 °C under orbital stirring at 100 rpm until the optical density measured at 600 nm reached 1 in a UV-Vis spectrometer (UV-530, Bonsai technologies, Spain). At this point, the bacteria from culture were collected by centrifugation (Labofuge 400 centrifuge, Thermo Scientific, USA) at 1500 rpm for 10 min at room temperature, washed three times with sterile phosphate buffer saline (PBS, Sigma-Aldrich, pH 7.4) and resuspended in 10 mL of PBS. The number of bacteria were retrospectively confirmed by plating serial dilutions of the starting inocula in agar Petri dishes (Tryptic Soy Agar, TSA 90 MM plate agar, BD Difco, USA). The chosen dilution was that allowing the direct counting of colony-forming units (CFUs). The chosen dilution was that of concentration 1.5 × 106 cells per mL. The different disk-shaped samples were soaked in 1 mL of bacterial suspension of 1.5 × 106 cells per mL and incubated at 37 °C under orbital stirring at 100 rpm for 90 min. Then, the samples were aseptically removed and rinsed three times with PBS to eliminate any free bacteria. Afterwards, two different experiments were carried out, which are described in the next sub-sections.
Data obtained from bacterial adhesion assays were expressed as means ± standard deviations of the independent experiments indicated in each case. Statistical analysis was performed using Statistical Package for the Social Sciences (SPSS) version 19 software. Statistical comparisons were made by analysis of variance (ANOVA). The Scheffé test was used for post hoc evaluation of differences among groups. In all statistical evaluations, p < 0.05 was considered as statistically significant.
In vitro cephalexin loading and release assays
Cephalexin (CPX) loading assays were performed by soaking 250 mg of powder samples in 10 mL of a 20 mM CPX solution in water (pH = 6) for 40 hours under magnetic stirring. Then, samples were filtered, gently washed with water and dried at room temperature under vacuum for further 24 hours. The presence of CPX in powdered samples was assessed by FTIR spectroscopy and the amount of drug loaded was determined by CHNS elemental chemical analysis.To carry out in vitro CPX release assays, 35 mg of each drug-loaded powder sample was compacted into disks of 6 mm in diameter and 2 mm in height by using uniaxial pressure at 2.75 MPa. Then, the disks were hung in screw caps using platinum wire and soaked into tubes containing 2 mL of PBS. The solution was kept at 37 °C and, to avoid limitations of the delivery rate by external diffusion constraints, continuous orbital stirring at 150 rpm was maintained during the assays. After determined periods of time, samples were removed from PBS and placed in tubes containing 2 mL of fresh PBS. The cumulative CPX released was determined by UV-Vis spectroscopy in a Unicam UV500 spectrometer (Gemini BV, Germany). For this purpose, absorbance was measured at 251 nm. CPX solutions in PBS with concentrations in the 0.01–0.2 mg mL−1 range were used for calibration. The curve was linear with a relationship of absorbance = 13.95 × [CPX] (correlation coefficient = 0.998). The apparent drug release was normalized to the amount of drug loaded within the samples to observe the relative amount of drug release. The CPX concentration was determined from the average of the readings from three different samples (N = 3), and data were presented as mean ± standard deviation.
Agar disk-diffusion tests of cephalexin-loaded materials
Agar disk diffusion tests (ADT) were used to examine the antibacterial effect of CPX-loaded materials. To carry out ADT, 20 mg of each CPX-loaded powder sample was compacted into disks of 6 mm diameter and 2 mm height by using uniaxial pressure at 2.75 MPa. For comparison, ADT were also performed using CPX-free disk-shaped samples. Prior to adhesion assay, samples were sterilized by UV irradiation for 7 min on each side of the piece. The surface of solid agar in Petri dishes was inoculated with a suspension of S. aureus bacterial culture with a cell concentration of 1.3 × 1010 bacteria per mL. Then, the disks were placed on the bacteria inoculated agar plates followed by incubation at 37 °C for 24 hours. After that, the bacterial inhibition zone size was measured as (outer diameter of the inhibition zone − disk diameter)/2.45 Each study was performed in triplicate.Results and discussion
Characterization of mesoporous materials
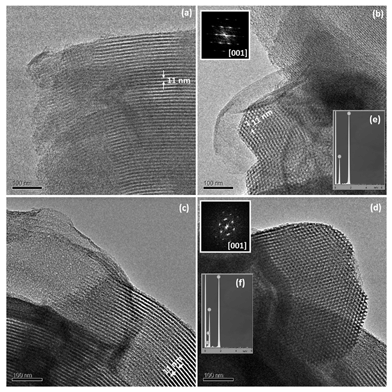
Structural characterization by XRD and TEM indicates that all samples exhibit ordered mesoporous arrangements typical of the SBA-15 structure (Fig. 1A and 2).31 XRD patterns at small angles exhibit well resolved peaks at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31/2
31/2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 d-spacing ratios, which can be indexed as 10, 11, and 20 reflections, respectively, of a 2D-hexagonal structure with p6mm plain group similar to that of SBA-15 (Fig. 1A). TEM images and their corresponding FT (Fast Fourier Transforms) for synthesized samples, acquired with the electron beam perpendicular and parallel to the cylindrical channels, confirm a honeycomb mesoporous arrangement typical of SBA-15 (Fig. 2). EDS analysis was performed during the TEM observation. Si and O peaks clearly appear for both samples (Fig. 2e and f) while the asymmetry on the O peak for SBA-Zwitter indicates the presence of nitrogen (Fig. 2f).
2 d-spacing ratios, which can be indexed as 10, 11, and 20 reflections, respectively, of a 2D-hexagonal structure with p6mm plain group similar to that of SBA-15 (Fig. 1A). TEM images and their corresponding FT (Fast Fourier Transforms) for synthesized samples, acquired with the electron beam perpendicular and parallel to the cylindrical channels, confirm a honeycomb mesoporous arrangement typical of SBA-15 (Fig. 2). EDS analysis was performed during the TEM observation. Si and O peaks clearly appear for both samples (Fig. 2e and f) while the asymmetry on the O peak for SBA-Zwitter indicates the presence of nitrogen (Fig. 2f).
 | ||
| Fig. 1 Characteristics of SBA-15 and SBA-Zwitter samples obtained by (A) XRD and (B) N2 adsorption measurements; the isotherm for sample SBA-Zwitter was vertically offset by 300 cm3 g−1 STP. | ||
N2 adsorption isotherms (Fig. 1B) can be identified as type IV isotherms according to the IUPAC classification, which are typical of mesoporous solids.34 The presence of H1 type hysteresis loops in the mesopore range indicates the existence of open-ended cylindrical mesopores with narrow pore size distributions, which are characteristic of SBA-15.34,36 The main textural features derived from the appropriate treatment of N2 adsorption data are summarized in Table 2. Both SBA-15 and SBA-Zwitter samples exhibit good textural properties, with high surface areas (SBET ∼ 500 m2 g−1), great pore volumes (VP ∼ 0.8 cm3 g−1) and large pore diameters (DP ∼ 9 nm). The slight decrease in the SBET, VP and DP values and the increase in the wall thickness (twall) of SBA-Zwitter compared to those of pure silica SBA-15 can be ascribed to the incorporation of organic functions into the mesoporous silica framework.
| Sample | S BET (m2 g−1) | V T (cm3 g−1) | V μP (cm3 g−1) | D P (nm) | a 0 (nm) | t wall (nm) | –(CH2)3NH(CH2)2NH2 per nm2 | SiOH per nm2 | IEP |
|---|---|---|---|---|---|---|---|---|---|
| a S BET is the surface area determined by using the BET method between the relative pressures (P/P0) 0.05 and 0.25. VP and VμP are, respectively, the total pore volume and micropore volume obtained using the t-plot method. The total pore volume was estimated from the amount of N2 adsorbed at a relative pressure of 0.97. DP is the pore diameter calculated by means of the BJH method from the adsorption branch of the isotherm. a0 is the unit cell parameter calculated by XRD, being a0 = 2/√3 × d10. twall is the wall thickness calculated using the equation twall = a0 − DP. The number of –(CH2)3NH(CH2)2NH2 groups was calculated on the basis of elemental chemical analysis. The number of silanol groups (SiOH) was determined by single pulse 29Si solid state NMR spectroscopy, as described in the experimental section. IEP point is the isoelectric point of samples determined by ζ-potential measurements. | |||||||||
| SBA-15 | 553 | 0.80 | 0.0021 | 9.8 | 11.9 | 2.1 | — | 10.9 | 4.2 ± 0.1 |
| SBA-Zwitter | 490 | 0.77 | ∼0 | 9.2 | 12.4 | 3.2 | 0.61 | 13.8 | 7.4 ± 0.1 |
Besides, SBA-Zwitter shows negligible micropore volume, which suggests that the grafted groups block such a contribution upon functionalization by lining the mesopore surface. The noticeable free mesopore area and pore volume indicate that these materials can be used as host matrices to load large amounts of biologically active molecules, such as drugs, peptides and relatively small proteins, for subsequent controlled release.23,46
Once the ordered mesoporous structure of these materials was confirmed, the chemical nature of the samples was investigated by using FTIR, CHNS elemental chemical analysis and STEM-EDS. Fig. 3 displays FTIR spectra of samples. As can be observed, all spectra show a broad band around 3400 cm−1 corresponding to the overlapping of the O–H stretching bands of hydrogen-bonded water molecules (H–O–H) and SiO–H stretching of surface silanols hydrogen bonded to molecular water (SiO–H⋯OH2).
The O–H bending vibration mode of these adsorbed water molecules is responsible for the band centered at 1630 cm−1. In addition, the Si–O in-plane stretching vibrations of the silanol Si–OH groups appear at 960 cm−1, whose reasonably high intensity accounts for the relatively low polymerization degree of the silica matrices, as is demonstrated by 29Si MAS solid state NMR study (vide infra). The intense silicon–oxygen covalent bond vibrations appearing in the 1100–1000 cm−1 range indicate the existence of a silica network, where oxygen atoms play the role of bridges between two silicon atoms. In addition, the symmetric stretching vibration of Si–O–Si and its bending mode appear at ca. 800 and 440 cm−1, respectively.47 The low energy band at 560 cm−1 is assigned to Si–O stretching of the silica network defects. In addition, several bands in the 2980–2850 cm−1 range, assigned to the C–H symmetric and antisymmetric stretching vibrations of –CH2– groups of the residual surfactant, are also distinguished. There are several vibration bands ascribed to bending vibrations of –CH2 groups of the template in the 1500–1330 cm−1 range. The relative intensity of such bands is higher in the spectrum of SBA-Zwitter than that of SBA-15 due to the presence of alkyl groups of DAMO. The FTIR spectrum of SBA-Zwitter displays additional bands at 3368 and 3248 cm−1 that are assigned to stretching vibrations of primary (NH2) and secondary (NH) amino groups, respectively. The strong absorption in the 3600–3400 cm−1 range appearing in the spectrum is attributable to the presence of water in the solid. The increase in the intensity and broadening of the band centered at 1630 cm−1 from 1680–1600 cm−1 for SBA-15 to 1680–1560 cm−1 for SBA-Zwitter points to the presence of a new band overlapping δHOH vibration of adsorbed water molecules. This band, centered at ca. 1580 cm−1 can be assigned to δNH, δNH2+, and δNH3+, although δCH2 bands of Si–CH2 groups of DAMO could also be present in the same interval.48 Moreover, at 1414 cm−1 there is a characteristic band of δNH2+ within the νCN band of DAMO. These two bands suggest that the SBA-Zwitter sample contains protonated amino groups, forming zwitterionic species via deprotonation of adjacent silanol groups (–SiO−).
TG–DTA and CHNS elemental analyses allowed determining the amount of residual surfactant, being less than 5% wt in both samples. This small amount of residual surfactant did not have any bactericidal effect, as derived from in vitro assays with S. aureus (vide infra). Elemental chemical analysis revealed that the number of functional groups present in the SBA-Zwitter sample was 0.61 [–(CH2)3NH(CH2)2NH2] per nm2, which is almost a half of the nominal value of 1.2 groups per nm2 that corresponds to the 0.90/0.10 TEOS/DAMO molar ratio used during the synthesis. Such finding points to an upper limit of functional groups from DAMO per square nanometer capable of being incorporated into the silica framework without provoking the loss of the mesostructural order. This is a well-known characteristic of mesoporous samples functionalized using the one-pot or co-condensation method.29 This fact was demonstrated by carrying out the functionalization of SBA-15 with DAMO via the co-condensation route and employing different TEOS/DAMO molar ratios from that used to synthesize SBA-Zwitter (results not shown). Thus, when using a 0.95/0.05 TEOS/DAMO molar ratio, 0.65 [–(CH2)3NH(CH2)2NH2] per nm2 were determined in the resulting SBA-15 type ordered mesoporous material. In contrast, when using a higher nominal functionalization degree (0.85/0.15 TEOS/DAMO molar ratio) the resulting sample lacked structural order. In this case the organic functions from DAMO would be somehow disrupting the silica framework and hindering the formation of ordered mesostructures.
To further investigate if there is a homogeneous distribution of N within mesoporous crystals, chemical mapping by means of EDS spectroscopy under STEM configuration of samples was carried out. The EDS spectrum of pure silica SBA-15 displays typical signals of Si and O. Fig. 4a shows the STEM image of SBA-Zwitter taken with the electron beam perpendicular to the mesochannels. The corresponding EDS spectrum displays two additional maxima that can be attributed to C and N from DAMO (Fig. 4b). An EDS study was carried out by selecting different mesoporous crystals and recording N, O and Si maps from appropriate Kα signals (Fig. 4c–e). Mapping of the square region highlighted in the STEM image shows a very weak N signal (previously observed in the punctual EDS analysis in Fig. 2), suggesting that N of [–(CH2)3NH(CH2)2NH2] groups from DAMO are randomly and homogeneously distributed within the mesoporous silica framework. This fact can be ascribed to the synthetic approach followed to functionalize SBA-15, i.e. co-condensation, as has been widely reported.29 These findings together with the results derived from FTIR and 13C NMR spectroscopy (vide infra) account for the homogeneous distribution of >NH2⊕⋯⊖OSi– and –NH3⊕⋯⊖OSi– zwitterionic species in the surface of the SBA-Zwitter sample.
29Si MAS-NMR measurements were performed to assess the chemical grafting of organosiloxane to the silica networks. Tetra-functional silicon centers were named with the conventional Qn notation where Q refers to [(SiO)nSi(OX)4−n] units, n being the number of bridging oxygen atoms surrounding the central silicon atom and X = H or CH2CH3. Similarly, tri-functional silicon centers were named with the conventional Tm notation where T refers to [(SiO)mRSi(OX)3−m] units, m being the number of bridging oxygen atoms surrounding the central silicon atom, X = H or CH3 and R = (CH2)3NH(CH2)2NH2 of DAMO. The relative populations of silicon environments were calculated by deconvolution of the 29Si NMR spectra into individual Gaussian peaks. All the resonance signals from the spectra and the corresponding relative peak areas are displayed in Table 3. Spectra of both samples display resonances at ca. −84, −93, −103 and −112 ppm assigned to silicon atoms in Q1, Q2, Q3 and Q4 environments (Fig. 5). The spectral analysis suggests that the surface Si–OH groups are associated as Q1, Q2 and Q3 structural units in pure-silica SBA-15. The relative high amount of silanol groups can be explained by the method used for the surfactant removal, i.e. solvent extraction, which leads to matrices with a lower condensation degree than those resulting from surfactant calcination.29 These finding agrees with the results derived from FTIR, where vibration bands of relatively high intensity due to silanol groups were observed in the spectra.
 | ||
| Fig. 5 29Si MAS NMR spectra of SBA-15 and SBA-Zwitter samples. The component peaks obtained from spectral deconvolutions are displayed by green dotted lines. | ||
The 29Si MAS solid state NMR spectrum of the SBA-Zwitter sample shows two additional down-field peaks assigned to organosiloxane species T2 at ca. −60 and T3 at ca. −69 ppm. This confirms the existence of covalent bonds between the silica surface and the organic groups, even if some degree of self-condensation cannot be overruled. The relative abundance of Tn species allowed us to calculate a molar functionalization degree (%F) of 6%, which is almost a half of the nominal one (10%). This result is in good agreement with the results derived from elemental chemical analysis discussed above, where the number of –(CH2)3NH(CH2)2NH2 groups per square nanometer in samples (0.61) were almost a half of the nominal value (1.2).
1H → 13C CP/MAS solid state NMR spectra were collected to confirm the functionalization of mesoporous materials and to get information about the ionization state and hydrogen bonding environment of amine groups from DAMO.
As expected, signals attributed to the residual surfactant are found in the 13C NMR spectrum of both pure silica SBA-15 and SBA-Zwitter materials (indicated by asterisks in Fig. 6), in agreement with results derived from elemental chemical analyses and TG–DTA measurements. The SBA-Zwitter spectrum exhibits several signals that can be assigned to the different carbon atoms from DAMO. Signals at 47.2 and 52.8 are indistinctly attributed to carbons C3 and C4 of DAMO that, being close to protonation centers (–NH2+– and –NH3+), may be present in different chemical environments originating multiple signals.49 The well-defined signals at 38.7, 21.5 and 11.2 ppm are respectively ascribed to C5, C2 and C1 carbons of DAMO. The resonance at 21.5 ppm, representing C2, exhibits a larger shift change toward upper field relative to pure DAMO (ca. 26 ppm) due to the protonation of amine groups in SBA-Zwitter.50 Nonetheless, a weak resonance at 25 ppm can be distinguished, which would correspond to the C2 carbon of non-protonated DAMO. These findings support the presence of >NH2⊕⋯⊖OSi– and –NH3⊕⋯⊖OSi– zwitterionic species in the SBA-Zwitter sample, although a very small population of amine groups could remain non-protonated.
Once the chemical nature of SBA-Zwitter was investigated, its surface charge as a function of pH was evaluated by recording ζ-potential measurements at different pH values. The resulting ζ-potential vs. pH plots are displayed in Fig. 7. The different equilibria accounting for the surface charges experimentally observed are displayed below:30
| –Si–OH + H2O ⇄ –Si–O− + H3O+ | (2) |
| –NH2 + H2O ⇄ –NH3+ + OH− | (3) |
| >NH + H2O ⇄ >NH2+ + OH− | (4) |
 | ||
| Fig. 7 ζ-Potential vs. pH plots of SBA-15 and SBA-Zwitter samples. The shaded area indicates the zero ζ-potential region for sample SBA-Zwitter. | ||
The isoelectric point (IEP) of SBA-15, which is strongly related to the zero surface charge point, is ca. 4.2, in agreement with the results reported elsewhere.11,30 The high number of silanol groups on the surface of SBA-15, 10.9 SiOH per square nanometer (Table 2), would be responsible for this behavior. Silanol groups, which exhibit weak acidic Brönsted character, would be deprotonated as –Si–O− groups at pH values above 4.2 (eqn (2)), providing a negative surface charge in the pH range of biological interest. On the other hand, SBA-Zwitter exhibits an IEP of ca. 7.4, which matches to the physiological pH. Below such a pH, the presence of protonated amino groups from DAMO (eqn (3) and (4)) provides a mesoporous material of positive surface charges. At pH 7.4 the concomitant presence of –Si–O− and protonated amino groups (>NH2+ and –NH3+) forming zwitterionic pairs results in a zero surface charge. Finally, at pH higher than 7.4 the greater number of silanol groups (13.8 SiOH per nm2) compared to that of diamine moieties (0.61 –(CH2)3NH(CH2)2NH2– groups per nm2) (Table 2) creates a net negative surface charge.
It has been demonstrated that SBA-Zwitter exhibits >NH2⊕/–SiO⊖ and –NH3⊕/–SiO⊖zwitterionic pairs on its surface. Moreover, ζ-potential measurements in aqueous media point to the preservation of the zwitterionic nature at the physiological pH of 7.4. The next step would be to evaluate the capability of SBA-Zwitter to inhibit bacterial adhesion compared to pure silica SBA-15. For this purpose, in vitro bacterial adhesion assays at pH 7.4 using S. aureus were carried out, at is discussed in the next section. Moreover, the good textural and distinctive chemical characteristics of SBA-Zwitter make this material an excellent candidate to host therapeutic molecules and release them in a sustained fashion. Therefore, the in vitro drug loading and release capabilities of SBA-Zwitter compared to SBA-15 were also evaluated, as is described below.
In vitro bacterial adhesion assays
To further evaluate the behavior of the SBA-Zwitter material towards bacterial adhesion, in vitro assays were carried out by soaking disk-shaped materials for 90 min at 37 °C in a suspension of S. aureus in PBS at pH of 7.4, as described in the experimental section. For comparison, in vitro bacterial adhesion assays using SBA-15 were also performed. With the aim of normalizing the number of CFUs per surface unit, the microstructure of disk-shaped samples was investigated. SEM micrographs of disk-shaped SBA-15 and SBA-Zwitter show the characteristic microstructure for SBA-15 type mesoporous materials, i.e. a material consisting of many rope-like domains with relatively uniform sizes of around 1 μm, which are aggregated into wheat-like macrostructures (Fig. S1, see ESI†).31 Hg intrusion porosimetry measurements reveal the presence of intergranular pores with sizes in the 100–600 μm and 5–600 μm ranges for SBA-15 and SBA-Zwitter, respectively. The total surface area within such ranges of macroporosity was 20 cm2 g−1 for SBA-15 and 160 cm2 g−1 for SBA-Zwitter, the higher porosity of the latter being attributable to the presence of organic moieties during the synthetic procedure. Therefore, the degrees of S. aureus adhesion for different material surfaces, expressed as CFUs per square centimetre, are displayed as histograms in Fig. 8. The adhered bacteria were ca. 2.88 ± 0.53 and (2.16 ± 0.66) × 103 CFUs per cm2 on SBA-Zwitter and SBA-15 samples, respectively. The statistic one-way ANOVA tests show that the bacterial adhesion values for the different surfaces were significantly different (p < 0.01). S. aureus adhesion on SBA-Zwitter samples significantly decreased ca. 99.9% compared to SBA-15 samples. This effect can be attributed to the presence of >NH2⊕/–SiO⊖ and –NH3⊕/–SiO⊖zwitterionic pairs on the material surface of SBA-Zwitter, which would provide its surface ultralow-fouling capability towards S. aureus bacteria adhesion. The mechanism of inhibition of bacterial adhesion on zwitterionic surfaces is not fully understood. However, it can be explained as an increase of surface hydration via electrostatic interactions in addition to hydrogen bonding due to great polarity of the charged functional groups.51 Thus, water molecules on the zwitterionic surface would create a strong repulsive force on the bacteria as they approach the surface.The visualization of adhered bacteria onto different surfaces was performed by confocal microscopy using a fluorescence based Live/Dead® Baclight™ bacterial viability test. This assay provides useful information about the membrane integrity of S. aureus. Fig. 8 shows images collected by confocal fluorescence microscopy after 90 minutes of bacterial adhesion assay onto disk-shaped SBA-15 and SBA-Zwitter surfaces. The surface of pure silica SBA-15, used as reference, is colonized by S. aureus bacteria with intact cell membranes, as derived from bright green fluorescence signals.
There are regions exhibiting significantly less green fluorescence, which could be due to the presence of bacteria with slightly damaged membranes. Nonetheless, red fluorescence is not observed, which indicates that most of the bacteria adhered remain viable on the surface of SBA-15, i.e. this material does not produce the bactericidal effect during the 90 min of adhesion assay. In contrast, the SBA-Zwitter surface exhibits minimal fluorescence (Fig. 8). In fact there are only very small regions where green fluorescence can be detected. The presence of >NH2⊕/–SiO⊖ and –NH3⊕/–SiO⊖zwitterionic pairs on the surface of SBA-Zwitter would hinder the adhesion of S. aureus. The small zones showing green fluorescence could be due to small regions where amino groups remain unprotonated, in agreement with results derived from 1H → 13C CP/MAS solid state NMR spectroscopy. The qualitative results derived from confocal microscopy support the quantitative results derived from CFU counting, where SBA-Zwitter inhibited up to a 99.9% amount of adhered bacteria compared to pure silica SBA-15.
In vitro antibiotic adsorption and release
To test the performance of SBA-Zwitter as a controlled delivery system, the zwitterionic antibiotic cephalexin (CPX) was selected and in vitro loading and release assays were performed. For comparison, in vitro CPX loading and delivery tests were performed in parallel using SBA-15 as the host matrix. Loading assays were carried out by soaking powdered samples in water (pH ∼ 6).52 Elemental chemical analyses were used to quantify the amount of CPX loaded into the different mesoporous samples. The results are shown in Table 4, 11.2 and 12.7 mg g−1 being the amounts of CPX loaded for SBA-15 and SBA-Zwitter, respectively. It should be noticed that the relatively small amount of CPX loaded, ∼1% in both materials, is not expected to modify the chemical nature of the host matrices. In fact, ζ-potential values at pH 7.4 of CPX-loaded samples were ca. 0 mV and −20 mV for SBA-Zwitter and SBA-15, respectively. These values are similar to those found for materials before CPX-loading. This fact is especially relevant in the case of SBA-Zwitter, where the zwitterionic nature of its surface needs to be preserved to inhibit bacterial adhesion.| Sample | W 0 (mg g−1) | k off ( × 103) (h−1) | ΔG (10−21 J) | K s (h−1) | R 2 |
|---|---|---|---|---|---|
| SBA-15 | 11.2 | 7.4 ± 3.0 | −7.5 ± 2.9 | 0.77 ± 0.34 | 0.994 |
| SBA-Zwitter | 12.7 | 1.51 ± 0.09 | −7.0 ± 0.9 | 0.18 ± 0.3 | 0.993 |
The main driving forces contributing to the adsorption of molecules on mesoporous silica can be regarded as electrostatic or Coulombic interaction, hydrogen bonding and non-polar interaction.30 Herein, the non-polar interaction may be overruled due to the hydrophilic nature of both CPX and mesoporous matrices synthesized in this work. In aqueous media, the CPX molecule exhibits different ionization states depending on the pH due to the presence of one carboxylic acid group and one amino group in its structure (Fig. 9).53 At the loading pH of 6, 86% of CPX is found as zwitterionic species (CPX±), where the amino group is protonated as –NH3+ and the carboxylic acid group is deprotonated as –COO−. Therefore, only 14% of CPX molecules would exist as positively charge species (CPX+). On the other hand, Fig. 6 provides information about the estimated net surface charge of mesoporous samples. Thus, at pH = 6 SBA-15 and SBA-Zwitter exhibit ζ-potential values of ca. −14 and +21 mV, respectively. Zwitterionic CPX± is capable of interacting through its protonated amino group with deprotonated silanol groups on the surface of SBA-15 via electrostatic interactions (–NH3⊕⋯⊖OSi–). On the other hand, since there are Si–OH groups remaining on the SBA-15 surface, hydrogen bond interactions with deprotonated carboxylic acid groups from CPX± (–COO⊖⋯HO–Si–) could take place. Besides this type of interaction, which is also present between CPX and SBA-Zwitter sample, additional host–guest attracting forces occurs. Thus, CPX± is capable of interacting with the positively charged SBA-Zwitter surface via electrostatic attracting interactions between protonated amino groups from DAMO and deprotonated carboxylic acid groups from CPX (>NH2⊕⋯⊖OOC– and –NH3⊕⋯⊖OOC–). These additional attractive electrostatic interactions would account for the slightly higher CPX loading capability of SBA-Zwitter than that of SBA-15, despite the higher surface area of the latter.
In vitro release tests were carried out in PBS at pH 7.4 and 37 °C. Fig. 10 shows CPX release patterns from both mesoporous matrices at the physiological pH of 7.4. Sustained drug release profiles are observed in all cases. For porous matrices it has been reported that drug release can be mediated by diffusion, erosion/degradation and swelling followed by diffusion.54
 | ||
| Fig. 10 Cephalexin (CPX) release profiles at pH 7.4 (phosphate buffer, PBS) for SBA-15 and SBA-Zwitter samples. Error bars represent the standard deviation for three measurements (N = 3). | ||
In fact, drug release is a combination of these three mechanisms with different modes of erosion: surface and bulk erosion. Although some matrix erosion and dissolution are involved, under perfect sink conditions, the main driving force for drug departure out of the mesoporous matrices is pore diffusion/convection, which can be fitted to first order kinetics. In addition to the diffusion-driven transport of drug molecules drug-carrier host–guest interactions are key factors to dictate drug release profiles.23,55 Drug molecules may directly interact with mesoporous materials, lowering their solubility and/or retarding their release. In this case, drug molecules are termed as associated, which need to be disassociated from nanocarriers prior to release. The association and dissociation processes are assumed to be reversible. Furthermore, the reversible association of a drug molecule with a carrier is assumed to follow first order kinetics. Therefore, the theoretical model adopted in this work considers first-order diffusion/convection and drug association/dissociation.55 Concretely, drug release patterns correspond to fast diffusion/convection but slow association/dissociation. This leads to a decoupling of drug association/dissociation from drug diffusion/convection: the fast release of initially free drug molecules via diffusion/convection and the slow release of initially bound drug molecules that is dictated by the dissociation process. Accordingly, two first order kinetics or two exponential release mechanisms can be described as follows:55
 | (5) |
The free energy difference between the free and bound states, ΔG, determines the amounts of initially free and bound drug, and can be calculated by the equation
 | (6) |
Fitting experimental release patterns to eqn (5) allowed us to determine the experimental values for ks, kon and koff. Then ΔG was calculated using eqn (6). The experimental results are summarized in Table 4. There is a good fitting of experimental results to the theoretical model (R2 > 0.99). The conditions ks ≫ kon and ks ≫ koff are fulfilled, which indicates that diffusion and convection are not neglected during the steady-state release. Accordingly, drug release profiles can be classified as low initial burst release with steady-state release.56
Let us analyse the meaning of the different parameters derived from the fitting of experimental data to the theoretical model, which are displayed in Table 4. ΔG gives us information about the magnitude of initial burst release. Fig. 10 shows a similar initial burst release of ca. 20% for both mesoporous materials. This is in very good agreement with the estimated value for ΔG, being ∼−7 × 10−21 J for both samples. The negative value of ΔG accounts for increasing CPX-mesoporous matrix association, which reduces initial burst release and enhances steady state. ks provides information about the rate of diffusion/convection, but not about the magnitude of the initial burst release. The model reveals a greater ks value for SBA-Zwitter (∼0.18 h−1) than that of SBA-15 (∼0.77 h−1), which is responsible for the prolonged initial burst release of the former. This indicates that CPX exhibits lower diffusivity in SBA-Zwitter than in SBA-15. Since the greater the pore diameter the faster the rate of drug diffusion from mesopores,23,57 SBA-15 (DP = 9.8 nm) permits higher CPX diffusivity than SBA-Zwitter (DP = 9.2) (Table 2). The carrier-drug host–guest interactions can be related to the koff value. Increasing koff enhances steady release following initial burst release, but has no effect on the magnitude of initial burst release. The koff value of SBA-Zwitter is almost 5-fold smaller (∼1.5 × 10−3 h−1) than that of SBA-15 (∼7.4 × 10−3 h−1) (Table 4). This is consistent with the existence of strong interactions between SBA-Zwitter and CPX, while weaker interactions occur between SBA-15 and CPX. At the physiological pH of 7.4, 80% of CPX exists in its anionic species (CPX−), whereas only 20% is present as the zwitterionic molecule (CPX±). At such a pH value, the surface charge of SBA-15 is negatively charged, whereas SBA-Zwitter is in the zwitterionic form. Thus, repulsive electrostatic interactions between deprotonated silanol groups in SBA-15 and CPX− bearing –COO− groups would predominate, which would trigger the CPX release from the mesoporous matrix. In contrast, electrostatic attractive interactions between primary and secondary protonated amino groups in SBA-Zwitter and –COO− groups from CPX− would account for more sustained drug release and a lower koff value for this functionalized material compared to pure silica SBA-15. Thus, after 15 days of assay almost 100% of CPX is released from SBA-15, while only 50% is released after the same time.
Agar disk-diffusion tests of cephalexin loaded materials
ADT allowed evaluating the capability of the CPX-loaded samples to eradicate S. aureus bacteria. CPX-free samples did not provoke inhibition zones during ADT, which accounts for their lack of bactericidal effect (Fig. S2, see ESI†). In contrast, well-defined inhibition zones were observed in the assays carried out with CPX-loaded samples. Thus, the inhibition zones were 5.6 ± 0.2 and 7.1 ± 0.4 mm for CPX-loaded SBA-15 and SBA-Zwitter samples, respectively. Such inhibition zones indicate that the amount of CPX diffused from both matrices during ADT exceeds the minimum inhibitory concentration (MIC) of S. aureus under these in vitro conditions. These results are in agreement with the in vitro delivery tests, where the amount of CPX released after 24 hours of assay is very similar in both samples (see Fig. 10). Note that the MIC value reported in the literature is in the 1–4 μg mL−1 range depending on the S. aureus collection strain.58 These results prove that the bactericidal capability of CPX is preserved after being subjected to the loading process into the different mesoporous samples.Conclusions
We have developed a bioceramic capable of decreasing relative bacterial adhesion from 100% to values lower than 0.1%, as derived from in vitro adhesion tests with S. aureus. This huge bacterial inhibition capability has not been previously reported for any mesoporous bioceramic at the physiological pH of 7.4. To attain this goal, the surface of SBA-15 has been provided with zwitterionic pairs (–NH3⊕/–SiO⊖ and >NH2⊕/–SiO⊖) by following the co-condensation route. A great antibiofouling capability is achieved due to the presence of very small amounts of functionalizing agent. This experimental result can be better understood thanks to current advanced techniques such as STEM-EDS, which permit the detection of almost negligible amounts of light elements such as nitrogen. In vitro loading and release assays using cephalexin as a model antibiotic prove that zwitterionic SBA-15 can host drugs into its mesopores and release them over long time periods (more than 15 days). These are remarkable results that provide significant insights into designing new bone implants capable of playing a dual role against infection. The zwitterionic nature permits inhibiting bacterial adhesion, which is the first stage of bone implant infection, whereas release of antibiotics would help eradicating bacteria in the surroundings of the implant site.Acknowledgements
This study was supported by research grants from Ministerio de Ciencia e Innovación (MICINN) through the projects MAT2012-35556 and CSO2010-11384-E (Agening Network of Excellence). Research by Marina Martínez-Carmona has also been supported by a PICATA predoctoral fellowship of the Moncloa Campus of International Excellence (UCM-UPM, ISCIII).Notes and references
- D. Campoccia, L. Montanaro and C. R. Arciola, Biomaterials, 2013, 34, 8018 CrossRef CAS PubMed.
- J. R. Edwards, K. D. Peterson, Y. Mu, S. Banerjee, K. Allen-Bridson, G. Morrell, M. A. Dudeck, D. A. Pollock and T. C. Horan, Am. J. Infect. Control, 2009, 37, 783 CrossRef PubMed.
- J. D. Whitehouse, N. D. Friedman, K. B. Kirkland, W. J. Richardson and D. J. Sexton, Infect. Control Hosp. Epidemiol., 2002, 23, 183 Search PubMed.
- D. Arcos, A. R. Boccaccini, M. Bohner, A. Díez-Pérez, M. Epple, E. Gómez-Barrena, A. Herrera, J. A. Planell, L. Rodríguez-Mañas and M. Vallet-Regí, Acta Biomater., 2014, 10, 1793 CrossRef CAS PubMed.
- C. E. Zobell, J. Bacteriol., 1943, 46, 39 CAS.
- J. Hasan, R. J. Crawford and E. P. Ivanova, Trends Biotechnol., 2013, 31, 295 CrossRef CAS PubMed.
- X. Ding, C. Yang, T. P. Lim, L. Y. Hsu, A. C. Engler, J. L. Hedrick and Y.-Y. Yang, Biomaterials, 2012, 33, 6593 CrossRef CAS PubMed.
- W. Heuer, A. Winkel, P. Kohorst, A. Lutzke, C. Pfaffenroth, H. Menzel, F. W. Bach, J. Volk, G. Leyhausen and M. Stiesch, Adv. Eng. Mater., 2010, 12, B609 CrossRef.
- S. Jiang and Z. Cao, Adv. Mater., 2010, 22, 920 CrossRef CAS PubMed.
- I. Eshet, V. Freger, R. Kasher, M. Herzberg, J. Lei and M. Ulbricht, Biomacromolecules, 2011, 12, 2681 CrossRef CAS PubMed.
- M. Colilla, I. Izquierdo-Barba, S. Sánchez-Salcedo, J. L. G. Fierro, J. L. Hueso and M. Vallet-Regí, Chem. Mater., 2010, 22, 6459 CrossRef CAS.
- I. Izquierdo-Barba, S. Sánchez-Salcedo, M. Colilla, M. J. Feito, C. Ramírez-Santillán, M. T. Portolés and M. Vallet-Regí, Acta Biomater., 2011, 7, 2977 CrossRef CAS PubMed.
- S. Sánchez-Salcedo, M. Colilla, I. Izquierdo-Barba and M. Vallet-Regí, J. Mater. Chem. B, 2013, 1, 1595 RSC.
- M. L. W. Knetsch and L. H. Koole, Polymers, 2011, 3, 340 CrossRef CAS PubMed.
- D. M. Eby, K. E. Farrington and G. R. Johnson, Biomacromolecules, 2008, 9, 2487 CrossRef CAS PubMed.
- I. Izquierdo-Barba, M. Vallet-Regí, N. Kupferschmidt, O. Terasaki, A. Schmidtchen and M. Malmsten, Biomaterials, 2009, 30, 5729 CrossRef CAS PubMed.
- S. B. Lee, R. R. Koepsel, S. W. Morley, K. Matyjaszewski, Y. Sun and A. J. Russell, Biomacromolecules, 2004, 5, 877 CrossRef CAS PubMed.
- M. Cicuéndez, I. Izquierdo-Barba, M. T. Portolés and M. Vallet-Regí, Eur. J. Pharm. Biopharm., 2013, 84, 115 CrossRef PubMed.
- D. Molina-Manso, M. Manzano, J. C. Doadrio, G. del Prado, A. Ortiz-Pérez, M. Vallet-Regí, E. Gómez-Barrena and J. Esteban, Int. J. Antimicrob. Agents, 2012, 40, 252 CrossRef CAS PubMed.
- M. Vallet-Regí, M. M. García and M. Colilla, Biomedical Applications of Mesoporous Ceramics. Drug Delivery, Smart Materials and Bone Tissue Engineering, CRC Press, Taylor & Francis, New York, USA, 2013 Search PubMed.
- D. Arcos and M. Vallet-Regí, Acta Mater., 2013, 61, 890 CrossRef CAS PubMed.
- M. Manzano, M. Colilla and M. Vallet-Regí, Expert Opin. Drug Delivery, 2009, 6, 1383 Search PubMed.
- M. Vallet-Regí, F. Balas and D. Arcos, Angew. Chem., Int. Ed., 2007, 46, 7548 CrossRef PubMed.
- M. Vallet-Regí and E. Ruiz-Hernández, Adv. Mater., 2011, 23, 5177 CrossRef PubMed.
- D. L. Slomberg, Y. Lu, A. D. Broadnax, R. A. Hunter, A. W. Carpenter and M. H. Schoenfisch, ACS Appl. Mater. Interfaces, 2013, 5, 9322–9329 CAS.
- Y. Lu, D. L. Slomberg, B. Sun and M. H. Schoenfisch, Small, 2013, 9, 2189–2198 CrossRef CAS PubMed.
- G. Cheng, H. Xue, Z. Zhang, S. Chen and S. Jiang, Angew. Chem., Int. Ed., 2008, 47, 8831 CrossRef CAS PubMed.
- Z. Cao, L. Mi, J. Mendiola, J.-R. Ella-Menye, L. Zhang, H. Xue and S. Jiang, Angew. Chem., Int. Ed., 2012, 51, 2602 CrossRef CAS PubMed.
- F. Hoffmann, M. Cornelius, J. Morell and M. Fröba, Angew. Chem., Int. Ed., 2006, 45, 3216 CrossRef CAS PubMed.
- A. Nieto, M. Colilla, F. Balas and M. Vallet-Regí, Langmuir, 2010, 26, 5038 CrossRef CAS PubMed.
- D. Zhao, J. Feng, Q. Huo, N. Melosh, G. H. Fredrickson, B. F. Chmelka and G. D. Stucky, Science, 1998, 279, 548 CrossRef CAS.
- W. Whitnall, T. Asefa and G. A. Ozin, Adv. Funct. Mater., 2005, 15, 1696 CrossRef CAS.
- S. Brunauer, P. H. Emmett and E. Teller, J. Am. Chem. Soc., 1938, 60, 309 CrossRef CAS.
- S. J. Gregg and K. S. W. Sing, Adsorption, surface area, and porosity, Academic Press, London, New York, 1982 Search PubMed.
- E. P. Barrett, L. G. Joyner and P. P. Halenda, J. Am. Chem. Soc., 1951, 73, 373 CrossRef CAS.
- M. Kruk, M. Jaroniec and A. Sayari, Chem. Mater., 1999, 11, 492 CrossRef CAS.
- B. H. Wouters, T. Chen, M. Dewilde and P. J. Grobet, Microporous Mesoporous Mater., 2001, 44–45, 453 CrossRef CAS.
- G. Cheng, Z. Zhang, S. Chen, J. D. Bryers and S. Jiang, Biomaterials, 2007, 28, 4192 CrossRef CAS PubMed.
- M. Katsikogianni and Y. F. Missirlis, Eur. Cells Mater., 2004, 8, 37 CAS.
- T. J. Kinnari, J. Esteban, N. Z. Martín-de-Hijas, O. Sánchez-Muñoz, S. Sánchez-Salcedo, M. Colilla, M. Vallet-Regí and E. Gómez-Barrena, J. Med. Microbiol., 2009, 58, 132 CrossRef CAS PubMed.
- T. J. Kinnari, J. Esteban, E. Gómez-Barrena, N. Zamora, R. Férnandez-Roblas, A. Nieto, J. C. Doadrio, A. López-Noriega, E. Ruiz-Hernández, D. Arcos and M. Vallet-Regí, J. Biomed. Mater. Res., Part A, 2009, 89, 215 Search PubMed.
- V. E. Wagner, J. T. Koberstein and J. D. Bryers, Biomaterials, 2004, 25, 2247 CrossRef CAS PubMed.
- E. W. Washburn, Proc. Natl. Acad. Sci. U. S. A., 1921, 7, 115 CrossRef CAS.
- L. Boulos, M. Prévost, B. Barbeau, J. Coallier and R. Desjardins, J. Microbiol. Methods, 1999, 37, 77 CrossRef CAS.
- S. Imazatoa, A. Kuramotoa, Y. Takahashia, S. Ebisua and M. C. Peters, Dent. Mater., 2006, 22, 527 CrossRef PubMed.
- M. Vallet-Regí, M. Colilla and B. González, Chem. Soc. Rev., 2011, 40, 596 RSC.
- C. J. Brinker, Sol–gel science: the physics and chemistry of sol–gel processing, Academic Press, Boston, 1990 Search PubMed.
- M. Colilla, A. J. Salinas and M. Vallet-Regí, Chem. Mater., 2006, 18, 5676 CrossRef CAS.
- M. Colilla, M. Darder, P. Aranda and E. Ruiz-Hitzky, J. Mater. Chem., 2005, 15, 3844 RSC.
- J. J. Yang, I. M. El-Nahhal, I. S. Chuang and G. E. Maciel, J. Non-Cryst. Solids, 1997, 209, 19 CrossRef CAS.
- S. Chen, L. Li, C. Zhao and J. Zheng, Polymer, 2010, 51, 5283 CrossRef CAS PubMed.
- M. S. Legnoverde, I. Jiménez-Morales, A. Jiménez-Morales, E. Rodríguez-Castellón and E. I. Basaldella, Med. Chem., 2013, 9, 672 CrossRef CAS.
- M. S. Legnoverde, S. Simonetti and E. I. Basaldella, Appl. Surf. Sci., 2014, 300, 37 CrossRef CAS PubMed.
- T. Ukmar, U. Maver, O. Planinšek, V. Kaučič, M. Gaberšček and A. Godec, J. Controlled Release, 2011, 155, 409 CrossRef CAS PubMed.
- L. Zeng, L. An and X. Wu, J. Drug Delivery, 2011, 2011, 15 Search PubMed.
- M. Ye, S. Kim and K. Park, J. Controlled Release, 2010, 146, 241 CrossRef CAS PubMed.
- M. Vallet-Regí, A. Rámila, R. P. del Real and J. Pérez-Pariente, Chem. Mater., 2001, 13, 308 CrossRef.
- J. M. Andrews, J. Antimicrob. Chemother., 2001, 48, 5 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: SEM micrographs and pore size distributions determined by Hg intrusion porosimetry for disk-shaped SBA-15 and SBA-Zwitter samples. See DOI: 10.1039/c4tb00690a |
| This journal is © The Royal Society of Chemistry 2014 |