Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Thermal stabilization of energetic materials by the aromatic nitrogen-rich 4,4′,5,5′-tetraamino-3,3′-bi-1,2,4-triazolium cation†
Thomas M.
Klapötke
*,
Philipp. C.
Schmid
,
Simon
Schnell
and
Jörg
Stierstorfer
Energetic Materials Research Department of Chemistry, University of Munich (LMU), Butenandtstr. 5-13, D-81377, Germany. E-mail: tmk@cup.uni-muenchen.de; Fax: +49 (0)89 2180 77492; Tel: +49 (0)89 2180 77491
First published on 25th November 2014
Abstract
4,4′,5,5′-Tetraamino-3,3′-bi-1,2,4-triazole (1) was prepared from readily available starting materials by a one-step procedure. Compound 1 consists of two combined aromatic triazole molecules with four amino moieties, resulting in a compound which has (i) high temperature stability, (ii) a high heat of formation, (iii) a high density and (iv) no sensitivity towards physical stimuli (friction, impact and electrostatic discharge). This compound has never previously been considered as a building block in the development of new energetic materials. We investigated compound 1 in detail as a potential nitrogen-rich, temperature-stable cation for the synthesis of energetic ionic derivatives (2–13) for use as environmentally benign explosives. The cation was combined with oxygen-rich counter-anions such as dinitramide (2), 5-nitrotetrazole-2-oxide (3), 5-nitrotetrazolate (4), nitrate (5), tetranitrobisimidazole (6), 5,5′-bitetrazole-1,1′-dioxide (7), 1,1′-dinitramino-5,5′-bitetrazolate (8), 5-nitriminotetrazolate (9), 1-methyl-5-nitriminotetrazolate (10), perchlorate (11), picrate (12) and nitroformate (13). Compounds 2–10 and 13 were characterized by low-temperature single-crystal X-ray diffraction. All the compounds were investigated by NMR and vibrational (IR, Raman) spectroscopy, mass spectrometry and elemental analysis. The excellent thermal properties of these compounds were determined by differential thermal analysis. The sensitivities towards impact, friction and electrical discharge were investigated using the BAM standards and a small-scale electrostatic discharge tester. The detonation parameters of the compounds without the inclusion of crystal water (1–3, 5–8, 11 and 13) were calculated using the EXPLO5 (V6.02) code and the calculated (CBS-4M) values for the enthalpy of formation.
Introduction
Research on energetic materials has mainly considered cyclic or caged nitramines – for example, 1,3,5-trinitro-1,3,5-triazinane (RDX) and 2,4,6,8,10,12-hexanitro-2,4,6,8,10,12-hexaazaisowurtzitane (CL-20) (Fig. 1).1 Since the development of RDX, any newly synthesized energetic compounds have had to compete with RDX, particularly in terms of the detonation pressure and detonation velocity, which are very important parameters in secondary explosives.2 Secondary explosives should also be stable with respect to temperature and have a high density, be safe to handle and cheap to synthesize. A high density is vital to the performance of an energetic material because the detonation pressure is proportional to the square of its density.2 In addition to enhanced physical properties, the demand for environmentally friendly nitrogen-rich energetic materials is steadily increasing. To reduce pollution of the environment through commonly used toxic and carcinogenic explosives such as RDX or lead azide, explosives that release mostly dinitrogen after decomposition are becoming increasingly important.3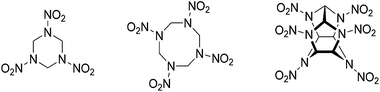 | ||
| Fig. 1 Chemical structure of the commonly used secondary explosives hexogen (RDX, left) and octogen (HMX, middle). CL-20 (right) is a high-performing alternative. | ||
One approach to synthesizing new energetic compounds is the preparation of energetic salts, which mostly have a high density and high stability as a result of their high lattice energy.2 As an alternative to using alkali metals (Na+, K+)4 as cations, nitrogen-rich cations have become more popular as a result of a potential hydrogen bond network, resulting in less sensitive materials,5 and a high positive heat of formation. This high positive heat of formation usually results in a high energetic performance as well as a good oxygen balance.6
Examples of commonly used nitrogen-rich cations are the guanidinium (G+), aminoguanidinium (AG+), diaminoguanidinium (DAG+), triaminoguanidinium (TAG+), ammonium (NH4+), hydroxylammonium (NH3OH+) and hydrazinium (N2H5+) ions. They vary considerably in their temperature stability and in their energetic performance. A common observed trend in simple nitrogen-rich cations is the decrease in temperature stability in the order G+, AG+, DAG+ and TAG+, whereas the performance increases with increasing numbers of energetic N–N bonds.7 The use of NH4+, NH3OH+ and N2H5+ cations is a valuable strategy to improve the energetic performance of explosives. Unfortunately, this usually leads to an increase in the mechanical sensitivity and a decrease in temperature stability from NH4+ over N2H5+ to NH3OH+.5,8 As a high thermal stability seems to be accompanied by a decrease in the energetic performance of energetic salts, the currently used nitrogen-rich salts are limited by either their thermal stability or by their energetic performance compared with RDX.
It therefore seems that, in many energetic materials, a good energetic performance and low sensitivity are mutually exclusive.8 This can be observed in the series of five-membered azoles from pyrazole to pentazole. Whereas pyrazole, which contains only two nitrogen atoms, has a low performance but high stability, a pentazole heterocyclic compound with five nitrogen atoms in the ring is too sensitive to be considered in any applications, but has a high performance.3a,f
Centore et al.9 reported the facile synthesis of 4,4′,5,5′-tetraamino-3,3′-bi-1,2,4-triazole (1). This compound has not yet been used as a building block in the development of new energetic materials, although it has been characterized by its decomposition point, elemental analysis and mass spectrometry. Detailed characterization using X-ray diffraction, elemental analysis and 13C NMR spectrometry, as well as the investigation of the energetic properties of compound 1, have not yet been reported. We report the synthesis of the temperature-stable nitrogen-rich salts of 4,4′,5,5′-tetraamino-3,3′-bi-1,2,4-triazole and a detailed investigation of the properties of the resulting energetic materials.
Results and discussion
Simple one-step synthesis
4,4′,5,5′-Tetraamino-3,3′-bi-1,2,4-triazole (1) was synthesized according to an improved version of a previously published procedure.9 By using phosphoric acid (100%) and phosphorus pentoxide, polyphosphoric acid is formed and can be used as a solvent. Diaminoguanidine monohydrochloride and oxalic acid were finely ground in a mortar and then added to the polyphosphoric acid. The temperature was kept at 120 °C overnight to form 1. The energetic salts were formed by simple metathesis reactions using compound 1 and different energetic anions, as illustrated in Scheme 1.4,4′,5,5′-Tetraamino-3,3′-bi-1,2,4-triazolium dinitramide 2, 4,4′,5,5′-tetraamino-3,3′-bi-1,2,4-triazolium dinitrotetrazolate-2N-oxide 3, 4,4′,5,5′-tetraamino-3,3′-bi-1,2,4-triazolium dinitrotetrazolate 4 and 4,4′,5,5′-tetraamino-3,3′-bi-1,2,4-triazolium di-1-methylnitriminotetrazolate dihydrate 10 were formed by a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric reaction of the respective anions, compound 1 and two equivalents of hydrochloric acid in water. The protonation of compound 1 leads to a better solubility in water. 4,4′,5,5′-Tetraamino-3,3′-bi-1,2,4-triazolium dinitrate 5, 4,4′,5,5′-tetraamino-3,3′-bi-1,2,4-triazolium diperchlorate 11, 4,4′,5,5′-tetraamino-3,3′-bi-1,2,4-triazolium dipicrate 12 and 4,4′,5,5′-tetraamino-3,3′-bi-1,2,4-triazolium dinitroformate 13 were formed by adding two equivalents of the anion to one equivalent of compound 1 under aqueous conditions. The nitrogen-rich compounds 4,4′,5,5′-tetraamino-3,3′-bi-1,2,4-triazolium tetranitrobis-imidazolate 6, 4,4′,5,5′-tetraamino-3,3′-bi-1,2,4-triazolium 5,5′-bitetrazole-1,1′-dioxide 7, 4,4′,5,5′-tetraamino-3,3′-bi-1,2,4-triazolium 1,1′-dinitramino-5,5′-bitetrazolate 8 and 4,4′,5,5′-tetraamino-3,3′-bi-1,2,4-triazolium 5-nitrimino-1H-tetrazolate hemihydrate 9 were formed by a 1
1 stoichiometric reaction of the respective anions, compound 1 and two equivalents of hydrochloric acid in water. The protonation of compound 1 leads to a better solubility in water. 4,4′,5,5′-Tetraamino-3,3′-bi-1,2,4-triazolium dinitrate 5, 4,4′,5,5′-tetraamino-3,3′-bi-1,2,4-triazolium diperchlorate 11, 4,4′,5,5′-tetraamino-3,3′-bi-1,2,4-triazolium dipicrate 12 and 4,4′,5,5′-tetraamino-3,3′-bi-1,2,4-triazolium dinitroformate 13 were formed by adding two equivalents of the anion to one equivalent of compound 1 under aqueous conditions. The nitrogen-rich compounds 4,4′,5,5′-tetraamino-3,3′-bi-1,2,4-triazolium tetranitrobis-imidazolate 6, 4,4′,5,5′-tetraamino-3,3′-bi-1,2,4-triazolium 5,5′-bitetrazole-1,1′-dioxide 7, 4,4′,5,5′-tetraamino-3,3′-bi-1,2,4-triazolium 1,1′-dinitramino-5,5′-bitetrazolate 8 and 4,4′,5,5′-tetraamino-3,3′-bi-1,2,4-triazolium 5-nitrimino-1H-tetrazolate hemihydrate 9 were formed by a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric reaction of the respective anions with compound 1 and two equivalents of hydrochloric acid in water.
1 stoichiometric reaction of the respective anions with compound 1 and two equivalents of hydrochloric acid in water.
X-ray diffraction
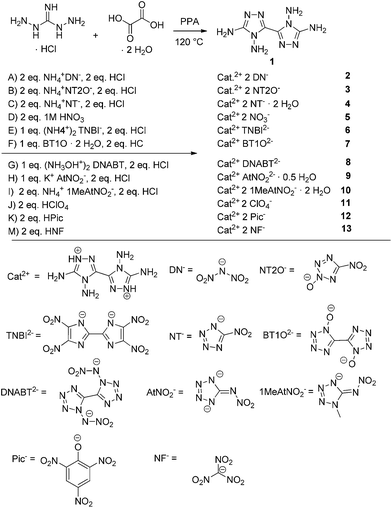
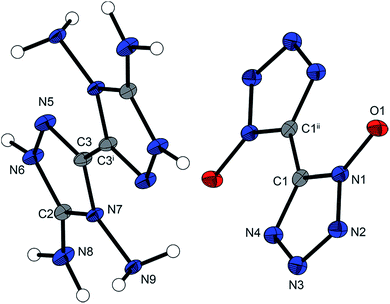
The crystal structures of compounds 2–10 and 13 were determined by low-temperature X-ray diffraction. Selected parameters of the X-ray determinations are given in Tables S1–S4 in the (ESI).† The cif files were deposited10 with CCDC nos 1029064 (2), 1029053 (3), 1029066 (4), 1029061 (5), 1029060 (6), 1029062 (7), 1029053 (7·2H2O), 1029058 (8), 1029056 (9), 1029055 (9a), 1029057 (9b), 1029054 (10) and 1029059 (13).4,4′,5,5′-Tetraamino-3,3′-bi-1,2,4-triazolium dinitramide (2) crystallizes from water in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) with a density of 1.860 g cm−3 at 173 K and one molecule per unit cell. The density of compound 2 is in the same range as previously reported dinitramides. For example, ammonium dinitramide with a density of 1.856 g cm−3 at 173 K11 or hydrazinium dinitramide (1.83 g cm−3, 298 K)12 show similar densities to the newly reported compound 2. The torsion angle of N3–C2–C2i–N1i is 0.5(2)°, showing that a nearly planar ring system is formed by the two triazoles. Through the aromaticity of the ring system, the triazoles form almost regular pentagons with angles near to 108° and with almost equal bond lengths between the ring atoms of 1.3–1.4 Å. The connecting C–C bond of the triazole rings with a length of 1.445(3) Å is significantly shorter than a C–C single bond (1.54 Å). Similar C–C bond lengths can be observed in the salts 3–10 and 13. Compared with the linking C–C bonds in other 1,2,4-triazoles – for example, 3,3′-dinitro-1,1′-dihydroxy-5,5′-bi-1,2,4-triazole and its derivatives with C–C bond lengths between 1.463(2) and 1.438(6) Å – the bond lengths in compounds 2–10 and 13 are very similar.8a,13 The distance C1–N5 is 1.317(2) Å and is thus remarkably shorter than the distance of the carbon to the nitrogen of the nitro group in 3,3′-dinitro-1,1′-dihydroxy-5,5′-bi-1,2,4-triazole and its derivatives, which have bond lengths between 1.454(5) and 1.427(5) Å.8a Moreover, the C1–N5 bond length is shorter than other C(triazole)–N(amino) distances (1.351(3) Å) of comparable compounds reported previously.14 Compounds 3–10 and 13 also show bond lengths around 1.31 Å for the equivalent C–N distance. Fig. 2 shows the molecular unit of compound 2 with selected bond lengths and selected bond angles in the caption.
with a density of 1.860 g cm−3 at 173 K and one molecule per unit cell. The density of compound 2 is in the same range as previously reported dinitramides. For example, ammonium dinitramide with a density of 1.856 g cm−3 at 173 K11 or hydrazinium dinitramide (1.83 g cm−3, 298 K)12 show similar densities to the newly reported compound 2. The torsion angle of N3–C2–C2i–N1i is 0.5(2)°, showing that a nearly planar ring system is formed by the two triazoles. Through the aromaticity of the ring system, the triazoles form almost regular pentagons with angles near to 108° and with almost equal bond lengths between the ring atoms of 1.3–1.4 Å. The connecting C–C bond of the triazole rings with a length of 1.445(3) Å is significantly shorter than a C–C single bond (1.54 Å). Similar C–C bond lengths can be observed in the salts 3–10 and 13. Compared with the linking C–C bonds in other 1,2,4-triazoles – for example, 3,3′-dinitro-1,1′-dihydroxy-5,5′-bi-1,2,4-triazole and its derivatives with C–C bond lengths between 1.463(2) and 1.438(6) Å – the bond lengths in compounds 2–10 and 13 are very similar.8a,13 The distance C1–N5 is 1.317(2) Å and is thus remarkably shorter than the distance of the carbon to the nitrogen of the nitro group in 3,3′-dinitro-1,1′-dihydroxy-5,5′-bi-1,2,4-triazole and its derivatives, which have bond lengths between 1.454(5) and 1.427(5) Å.8a Moreover, the C1–N5 bond length is shorter than other C(triazole)–N(amino) distances (1.351(3) Å) of comparable compounds reported previously.14 Compounds 3–10 and 13 also show bond lengths around 1.31 Å for the equivalent C–N distance. Fig. 2 shows the molecular unit of compound 2 with selected bond lengths and selected bond angles in the caption.
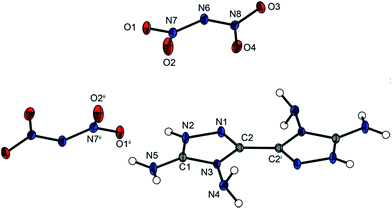
The anhydrous crystal structure of 4,4′,5,5′-tetraamino-3,3′-bi-1,2,4-triazolium dinitrotetrazolate-2N oxide (3) is described by the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) with a density of 1.833 g cm−3 at 173 K and one molecule per unit cell. Compound 3 almost reaches the density of the highly energetic hydroxylammonium nitrotetrazolate-N-oxide with a density of 1.850(2) g cm−3.8c In relation to the nitrogen-rich guanidinium, aminoguanidinium or diaminoguanidinium nitrotetrazolate-2N-oxides with densities of 1.6978(4), 1.697(2) and 1.6867(3) g cm−3, respectively, which are more stable than the hydroxylammonium salt, the density of 3 is significantly higher.8c The torsion angle of N9–C3–C3i–N6i is 0°, making the bicyclic ring system completely planar, similar to that of the cation in compound 2. The angles of the ring system and the bond lengths behave in the same way as described for compound 2. Likewise, the bond lengths and bond angles of the anion correspond to previously reported data.5 The molecular unit is shown in Fig. 3, with selected bond lengths and bond angles in the caption.
with a density of 1.833 g cm−3 at 173 K and one molecule per unit cell. Compound 3 almost reaches the density of the highly energetic hydroxylammonium nitrotetrazolate-N-oxide with a density of 1.850(2) g cm−3.8c In relation to the nitrogen-rich guanidinium, aminoguanidinium or diaminoguanidinium nitrotetrazolate-2N-oxides with densities of 1.6978(4), 1.697(2) and 1.6867(3) g cm−3, respectively, which are more stable than the hydroxylammonium salt, the density of 3 is significantly higher.8c The torsion angle of N9–C3–C3i–N6i is 0°, making the bicyclic ring system completely planar, similar to that of the cation in compound 2. The angles of the ring system and the bond lengths behave in the same way as described for compound 2. Likewise, the bond lengths and bond angles of the anion correspond to previously reported data.5 The molecular unit is shown in Fig. 3, with selected bond lengths and bond angles in the caption.
The energetic compound 4,4′,5,5′-tetraamino-3,3′-bi-1,2,4-triazolium dinitrate (5) crystallizes anhydrously from water in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) with one molecule per unit cell and a density of 1.779 g cm−3 at 173 K. The density of compound 5 is lower than those of compounds 2 and 3. Compared with hydroxylammonium nitrate with a density of 1.841 g cm−3,15 compound 5 has a lower density, whereas compound 5 has a higher density than guanidinium nitrate (1.410 g cm−3).16 With a torsion angle of 1.4(2)° of the plane N1–C1–C1i–N3i, the two triazoles are tilted only slightly more towards each other than in compounds 2–4. The bond angles and bond lengths of the bicyclic ring system match the reported data. Fig. 4 shows the molecular unit of compound 5.
with one molecule per unit cell and a density of 1.779 g cm−3 at 173 K. The density of compound 5 is lower than those of compounds 2 and 3. Compared with hydroxylammonium nitrate with a density of 1.841 g cm−3,15 compound 5 has a lower density, whereas compound 5 has a higher density than guanidinium nitrate (1.410 g cm−3).16 With a torsion angle of 1.4(2)° of the plane N1–C1–C1i–N3i, the two triazoles are tilted only slightly more towards each other than in compounds 2–4. The bond angles and bond lengths of the bicyclic ring system match the reported data. Fig. 4 shows the molecular unit of compound 5.
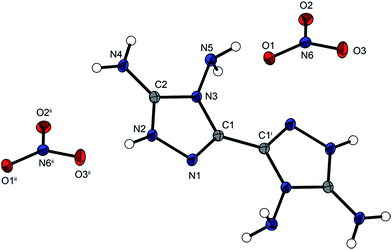 | ||
| Fig. 4 Molecular unit of 5. Ellipsoids are drawn at the 50% probability level. Selected bond length (Å): C1–C1i 1.454(6), C2–N4 1.318(8). | ||
The anhydrous salt 4,4′,5,5′-tetraamino-3,3′-bi-1,2,4-triazolium tetranitro-bisimidazolate (6) crystallizes in the monoclinic space group P21/c with two molecules per unit cell. Compound 6 has a high density of 1.879 g cm−3 at 173 K. The high density may be rationalized as being a result of the strong intra- and intermolecular interactions that arise through the hydrogen bond network formed by the combination of the four amino groups of the cation and the four nitro groups of the anion, resulting in very long hydrogen bridges. Only the hydrazinium salt of tetranitrobisimidazolate, with a density of 1.826 g cm−3 (298 K),17 showed a density in the range of compound 6; the other nitrogen-rich salts such as the guanidinium (ρ = 1.701 g cm−3 at 298 K) or the aminoguanidinium salt (ρ = 1.698 g cm−3 at 298 K) clearly have lower densities.17 The torsion angles of the two bicyclic ring systems show that two nearly planar moieties are formed. The C–C bond of the anion linking the two rings is in the same dimension as the bond of the cation and is comparable with the bond lengths of other tetranitrobisimidazoles.18Fig. 5 shows the molecular unit of compound 6 with selected torsion angles and hydrogen bond lengths in the caption.
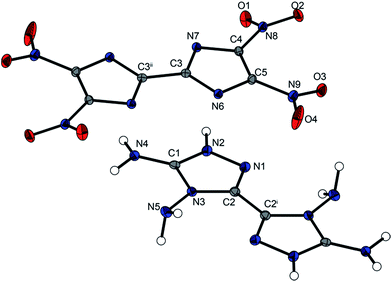
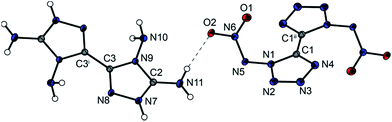
4,4′,5,5′-Tetraamino-3,3′-bi-1,2,4-triazolium 5,5′-bitetrazole-1,1′-dioxide (7) forms anhydrous crystals as well as crystals containing two water moieties when crystallized from water (7·2 H2O). The water-free compound 7 crystallizes in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) with a density of 1.686 g cm−3 at 173 K and one molecule per unit cell. The structure of the di-anion has been discussed previously.19,20 Compared with other energetic salts of 5,5′-bitetrazole-1,1′-dioxide, e.g. the hydroxylammonium salt TKX-50 (ρ173K = 1.915 g cm−3), the density of compound 7 is fairly low.19 However, compared with the thermally stable diguanidinium salt 5,5′-bitetrazole-1,1′-dioxide with a density of 1.639 g cm−3,20 compound 7 shows a slightly higher density. Fig. 6 shows the molecular unit of 7, with selected bond lengths and torsion angles in the caption. The molecular unit of 7·2H2O is illustrated in Fig. S2 in the ESI.†
with a density of 1.686 g cm−3 at 173 K and one molecule per unit cell. The structure of the di-anion has been discussed previously.19,20 Compared with other energetic salts of 5,5′-bitetrazole-1,1′-dioxide, e.g. the hydroxylammonium salt TKX-50 (ρ173K = 1.915 g cm−3), the density of compound 7 is fairly low.19 However, compared with the thermally stable diguanidinium salt 5,5′-bitetrazole-1,1′-dioxide with a density of 1.639 g cm−3,20 compound 7 shows a slightly higher density. Fig. 6 shows the molecular unit of 7, with selected bond lengths and torsion angles in the caption. The molecular unit of 7·2H2O is illustrated in Fig. S2 in the ESI.†
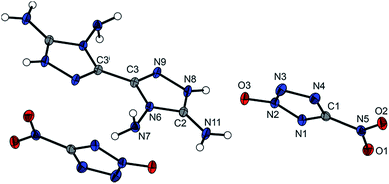
The anhydrous energetic compound 4,4′,5,5′-tetraamino-3,3′-bi-1,2,4-triazolium 1,1′-dinitramino-5,5′-bitetrazolate (8) crystallizes from water in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) with a density of 1.778 g cm−3 and one formula unit per unit cell. Dipotassium 1,1′-dinitramino-5,5′-bitetrazolate with a density of 2.172 g cm−3 is the only previously reported compound containing this anion.4a The anion of 8 has a larger torsion angle than the corresponding cation, forming a planar cation and a slightly tilted anion. The molecular unit of compound 8 is shown in Fig. 7.
with a density of 1.778 g cm−3 and one formula unit per unit cell. Dipotassium 1,1′-dinitramino-5,5′-bitetrazolate with a density of 2.172 g cm−3 is the only previously reported compound containing this anion.4a The anion of 8 has a larger torsion angle than the corresponding cation, forming a planar cation and a slightly tilted anion. The molecular unit of compound 8 is shown in Fig. 7.
The crystal structures of compounds 4, 7·2H2O, 9–10 and 13 were also determined and are presented in the ESI.†
Thermal analysis and compatibility
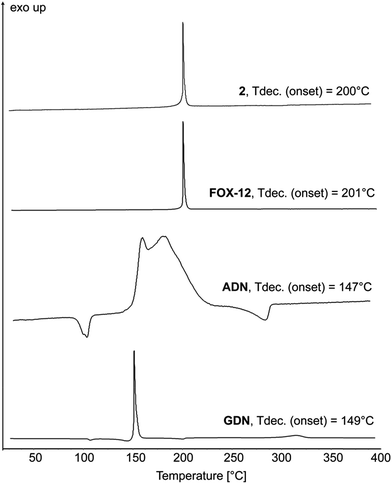
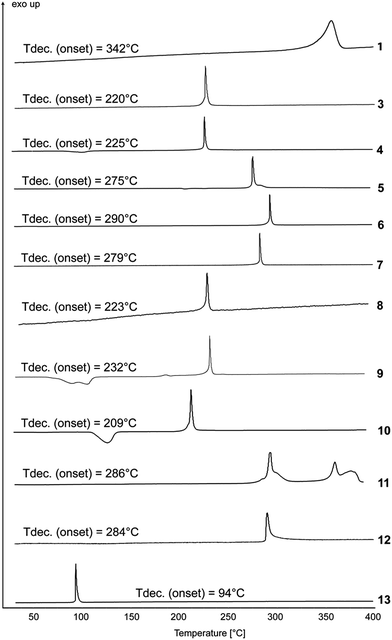
To identify the decomposition temperatures of compounds 1–13, differential thermal analysis (DTA) with a heating rate of 5 °C min−1 was used. The results are shown in Fig. 8 and 10.The decomposition temperature of the neutral compound 1 is very high (342 °C) and thus exceeds the decomposition temperature of the explosives RDX (Tdec. = 204 °C)21 and hexanitrostilbene (Tdec. = 316 °C).22 The dinitramide 2 decomposes at 200 °C. Dinitramide-based ionic energetic materials often lack thermal stability. One exception is FOX-12. Its decomposition temperature of 215 °C has been reported at a heating rate of 10 °C min−1.23 For better comparison of 2 with FOX-12, ammonium dinitramide (ADN) and guanidinium dinitramide (GDN), these compounds were re-measured on our instrument at a heating rate of 5 °C min−1 (Fig. 8). FOX-12 showed an onset decomposition temperature of 201 °C, which is virtually the same temperature as compound 2. GDN and ADN had lower decomposition temperatures of 149 and 147 °C. Table 1 compares the thermal stability (although a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 dinitramide salt) with other nitrogen-rich dinitramides.
2 dinitramide salt) with other nitrogen-rich dinitramides.
| Dinitramide | T dec. (onset) (°C) |
|---|---|
| a DN = dinitramide, A = ammonium, G = guanidinium, TAG = triaminoguanidinium, 1,5-DAT = 1,5-diaminotetrazolium, 1-Me-AT = 1-methyl-5-aminotetrazolium, 2-Me-AT = 2-methyl-5-aminotetrazolium, 5-AT = 5-aminotetrazolium, Tz = tetrazolium, 3,5-DATr = 3,5-diaminotriazolium, 3,6-DHyTT = 3,6-dihydrazino-tetrazinium. | |
| 2 | 200 |
| FOX-12 | 201 (215 at 10 °C min−1) (ref. 23) |
| ADN | 147 |
| GDN | 149 |
| TAG DN | 180 (ref. 27a) |
| 1,5-DAT DN | 135 (ref. 27b) |
| 1-Me-AT DN | 145 (ref. 27b) |
| 2-Me-AT DN | 148 (ref. 27c) |
| 5-AT DN | 117 (ref. 27c) |
| Tz DN | 110 (ref. 27c) |
| 3,5-DATr DN | 164 (ref. 27d) |
| 3,6-DHyTT DN | 152 (ref. 27e) |
Compounds 4, 9 and 10, containing two water moieties each, dehydrate at 89, 93 and 104 °C, respectively. The highest decomposition temperature of the energetic salts of compound 1 was measured for compound 6 (Tdec = 290 °C). After compound 6, compounds 5, 7, 11 and 12 also showed very high thermal stabilities, with onset decomposition temperatures of 275, 279, 286 and 284 °C, respectively. Compared with 1-methylnitriminotetrazolate (10), which shows a fairly low onset of decomposition at 209 °C, nitriminotetrazolate (9) has a higher onset of decomposition at 232 °C. This agrees with the decomposition temperatures of reported nitrogen-rich 1-methylnitriminotetrazolates with onset temperatures of around 210 °C.24
The nitrotetrazolate 4 decomposes at a temperature of 225 °C and thus shows a higher thermal stability than all other reported non-metal nitrotetrazolate salts, with guanidinium nitrotetrazolate being the most temperature-stable (Tdec. = 212 °C).5 Thermal stabilization of the anion in compound 8 could be achieved through cation metathesis. Although the potassium salt K2DNABT decomposes at 200 °C,4a the energetic compound 8 is thermally stable up to a temperature of 223 °C. Compared with the nitrogen-rich salts, only the ammonium salt (NH4)2DNABT lies in the range of the thermal stability of compound 8, whereas the hydroxylammonium salt (NH3OH)2DNABT has a much lower decomposition temperature of 170 °C.25 The onset decomposition temperature of nitrotetrazolate-2N-oxide (3) is 220 °C, showing a much higher thermal stability than other comparable nitrotetrazolate-2N-oxides, such as the guanidinium salt (Tdec. = 211 °C), the aminoguanidinium salt (Tdec. = 185 °C), the diamino-guanidinium salt (Tdec. = 174 °C), the triaminoguanidinium salt (Tdec. = 153 °C) or the ammonium salt (Tdec. = 173 °C).8c Compared with RDX, with a decomposition temperature of 204 °C,21 salts 2–12 have at least an equivalent thermal stability. Only the nitroformate 13 decomposes at 94 °C, which is slightly lower than the ammonium (116 °C), guanidinium (113 °C) and triaminoguanidinium (105 °C) salt, but higher than the corresponding aminoguanidinium (71 °C) and diaminoguanidinium (82 °C) salt.26
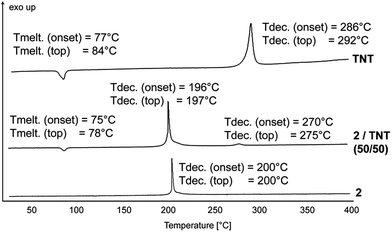
All explosives have to be coated for practical applications. A prominent mixture of RDX and TNT (ca. 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40) is called composition B. A compatibility test of compound 2 with TNT revealed that the decomposition temperature of the 2/TNT mixture was virtually the same as for pure compound 2 with respect to both the onset and the top of the peak. It can be concluded that dinitramide 2 is compatible with TNT (Fig. 9).
40) is called composition B. A compatibility test of compound 2 with TNT revealed that the decomposition temperature of the 2/TNT mixture was virtually the same as for pure compound 2 with respect to both the onset and the top of the peak. It can be concluded that dinitramide 2 is compatible with TNT (Fig. 9).
Sensitivities
Compounds 1–13 were tested for their sensitivity towards friction and impact using the BAM methods as described in the ESI.† Compounds 1–13 showed a wide range of sensitivities towards impact. Although the neutral compound 1 and compounds 6, 7 and 9 can be classified as insensitive towards impact (<40 J), compounds 2, 3, 8 and 11 show very high sensitivities from 6 J (3) to up to 3 J (8). The dinitramide 2, with an impact sensitivity of 5 J, is in the range of sensitivities towards impact observed for other nitrogen-rich dinitramides (ADN, 5 J;28 TAG DN, 2 J).27a The impact sensitivity of compound 3 (6 J) is comparable with the impact sensitivities of the corresponding hydroxylammonium or ammonium salts with impact sensitivities of 4 and 7 J, respectively, but does not reach the stability of the diaminoguanidnium nitrotetrazolate-2N-oxide (20 J) or the guanidinium salt, which is insensitive.8c Compound 8 shows the highest sensitivity towards impact stimuli with 3 J, which is a result of the highly sensitive anion. The corresponding hydroxylammonium and ammonium salts have an impact sensitivity of 2 J and thus lie in the same range as compound 8.25Compound 13 shows a sensitivity of 4 J. Compounds 4, 10 and 12 are fairly insensitive, with sensitivities >30 J. Compared with previously reported nitrogen-rich salts of 1-methyl-5-nitrimino-tetrazole, an impact sensitivity of up to 35 J is very high, especially as the aminoguanidinium or triaminoguanidinium salts have values of about 10 J.24 The nitrate salt 5 (15 J) has a moderate sensitivity towards friction. The compounds 2, 3, 8, 11 and 13 thus at least lie in the range of RDX (7.5 J)29 or have higher stabilities towards impact.
Except for compounds 8 and 11, the energetic salts and the neutral compound 1 are insensitive towards friction (360 N). The friction insensitivity of the dinitramide compound 2 is notable, as previously reported nitrogen-rich salts, such as triaminoguanidinium dinitramide (24 N)27a or ammonium dinitramide (72 N),28 have high sensitivities towards friction. In addition, the friction insensitivity of the nitrotetrazolate-2N-oxide 3 far exceeds the values reported for other nitrogen-rich nitrotetrazolate-2N-oxides, which range from guanidinium nitrotetrazolate-2N-oxide (252 N) to the hydroxylammonium salt with a friction sensitivity of 60 N.8c The perchlorate (11) and nitroformate (13) salts have a slightly higher sensitivity towards friction stimuli (240 and 160 N). Compounds 1–7 and 9–12 have a much lower sensitivity towards friction than RDX (FSRDX = 120 N).29 The ESD measurements show that compounds 1–7 and 9–13 are less sensitive towards electrostatic discharge (1, 5, 7 and 9 = 1.5 J; 2, 3 and 13 = 0.8 J; 12 = 0.75 J; 11 = 0.6 J; 10 = 0.5 J; 6 = 0.4 J; 4 = 0.3 J) than the sensitive compound 8 (0.05 J) or RDX (0.2 J).
Energetic performance
The enthalpies of formation were calculated using the atomization method with electronic energies (CBS-4M method) at room temperature (see ESI†). The heats and energies of formation for compounds 1, 3, 5–8, 11 and 13 are given in Table 2.| 1 | 2 | 3 | 5 | 6 | 7 | 8 | 11 | 13 | RDX | FOX-12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| a Impact sensitivity (BAM drop hammer, 1 of 6). b Friction sensitivity (BAM friction tester, 1 of 6). c Electrostatic discharge device (OZM). d Nitrogen content. e Oxygen balance. f Decomposition temperature from DSC (β = 5 °C). g Recalculated from low-temperature X-ray densities (ρ298K = ρT/(1 + αV(298 − T0); αV = 1.5 10−4 K−1). h Values in parentheses are the density obtained from the X-ray measurements at 298 K. i Calculated (CBS-4M) heat of formation. j Calculated energy of formation. k Energy of explosion. l Explosion temperature. m Detonation pressure. n Detonation velocity. o Assuming only gaseous products. | |||||||||||
| Formula | C4H8N10 | C4H10N16O8 | C6H10N20O6 | C4H10N12O6 | C10H10N18O8 | C6H10N18O2 | C6H10N22O4 | C4H10N10O8Cl2 | C6H10N16O12 | C3H6N6O6 | C2H7N7O5 |
| FW (g mol−1) | 196.17 | 410.22 | 458.11 | 322.20 | 510.30 | 366.26 | 454.29 | 397.09 | 498.24 | 222.12 | 209.12 |
| ISa (J) | 40 | 5 | 6 | 15 | 40 | 40 | 3 | 5 | 4 | 7.5 | 30 |
| FSb (N) | 360 | 360 | 360 | 360 | 360 | 360 | 10 | 240 | 160 | 120 | 350 |
| ESDc (J) | 1.5 | 0.8 | 0.8 | 1.5 | 0.4 | 1.5 | 0.05 | 0.9 | 0.8 | 0.20 | 1.5 |
| N (%) | 71.40 | 54.63 | 61.13 | 52.17 | 49.41 | 68.84 | 67.83 | 35.27 | 44.98 | 37.84 | 46.89 |
| Ωe (%) | −97.87 | −19.50 | −38.40 | −34.76 | −53.30 | −65.52 | −45.79 | −16.11 | −16.06 | −21.61 | −19.13 |
| T dec. (°C) | 342 | 200 | 220 | 275 | 290 | 279 | 223 | 286 | 94 | 204 | 201 |
| ρ (g cm−3) (298 K) | 1.68 (pyc.) | 1.826 (1.819)h | 1.799 | 1.746 | 1.844 | 1.655 | 1.745 | 1.870 | 1.837 | 1.806 | 1.754 |
| ΔfH°i (kJ mol−1) | 472.0 | 301.5 (302.8)h | 761.2 | −153.2 | 20.9 | 597.4 | 1107.7 | −23.1 | 173.4 | 70.3 | −355.0 |
| ΔfU°j (kJ kg−1) | 2520.3 | 837.7 (840.9)h | 1758.3 | −367.7 | 258.6 | 1733.7 | 2536.6 | 35.4 | 442.5 | 417.0 | −1585.0 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||||
| EXPLO V6.02 values: | |||||||||||
| –ΔEU°k (kJ kg−1) | 3101 | 4955 (4956)h | 4696 | 3871 | 3533 | 3262 | 4672 | 4589 | 5226 | 5845 | 3694 |
| T E (K) | 2088 | 3407 (3422)h | 3293 | 2790 | 2668 | 2446 | 3266 | 3492 | 3642 | 3810 | 2703 |
| p C–J (kbar) | 258 | 338 (338)h | 306 | 260 | 260 | 221 | 288 | 299 | 343 | 345 | 265 |
| D (m s−1) | 8944 | 9053 (9022)h | 8857 | 8334 | 8237 | 8081 | 8804 | 8290 | 8879 | 8861 | 8323 |
| V 0 (L kg−1) | 812 | 843 (843)h | 822 | 867 | 736 | 819 | 833 | 792 | 780 | 785 | 893 |
The detonation parameters of compounds 1–3, 5–8, 11 and 13 were calculated using the program package EXPLO5 (version 6.02).30 The program is based on the chemical equilibrium steady-state model of detonation. It uses the Becker–Kistiakowsky–Wilson equation of state for gaseous detonation products and Cowan–Fickett's equation of state for solid carbon. For these calculations, the low-temperature X-ray densities were converted to room temperature values with the equation ρ298K = ρT/(1 + αV(298 − T0); αV = 1.5 × 10−4 K−1.31 To verify this approximation, crystals of compound 2 were measured at 100, 173 and 298 K (see ESI†). The measured X-ray density of 1.819 g cm−3 (298 K) is almost the same as the recalculated density of 1.826 g cm−3 (298 K). The marginal influence on the calculated detonation performances is shown in Table 2.
The calculated detonation parameters are summarized in Table 2 and compared with the values calculated for FOX-12 and RDX. The energetic performances of compounds 6–8, 11 and 13 are discussed in detail in the ESI.†
Compared with the energetic salts, compound 1 has a fairly high heat of formation of 472 kJ mol−1. The dinitramide 2 has a heat of formation of 302 kJ mol−1. Compared with ADN (heat of formation −150 kJ mol−1), or hydroxylammonium dinitramide (heat of formation −34 kJ mol−1), the calculated heat of formation of compound 2 is significantly higher.12 A higher value is also obtained compared with RDX and FOX-12, which have heats of formation of 70 and −355 kJ mol−1, respectively. Compound 3 has a highly positive heat of formation of 761 kJ mol−1.
For neutral compound 1, a fairly high detonation velocity of 8944 m s−1 and a detonation pressure of 285 kbar were calculated. The highest detonation velocity of 9053 m s−1 in this work was calculated for dinitramide 2. This was also higher than that of RDX (8861 m s−1) and FOX-12 (8323 m s−1). When comparing the detonation pressures, compound 2 showed a value in the range of RDX (345 kbar) and a much higher value than FOX-12 (265 kbar). Compared with RDX, compound 3 had a very similar detonation velocity of 8857 m s−1 and a slightly lower detonation pressure of 306 kbar. The corresponding hydroxylammonium salt had a higher detonation velocity (9499 m s−1) and detonation pressure (410 kbar).8c The other reported nitrotetrazolate-2N-oxides had detonation parameters in the range of compound 3 (NH4NT2O pC–J = 322 kbar, D = 8885 m s−1) or had lower values (the G+, AG+, DAG+, TAG+ salts).8c The nitrate 5 had a detonation velocity of 8334 m s−1 and a detonation pressure of 260 kbar. The lowest detonation pressure (221 kbar) and velocity (8081 m s−1) of the compounds were for compound 7.
A small-scale reactivity test (SSRT; see ESI† for detailed setup) was conducted to assess the explosive performance of 2 compared with RDX and FOX-12. From measuring the volumes of the dents (Table 3), it can be concluded that the small-scale explosive performance of 2 is slightly lower than that of commonly used RDX, but far exceeds that of FOX-12.
| SSRT | ||
|---|---|---|
| Compound | Weight (mg) | Dent (mg SiO2) |
| 2 | 524 | 772 |
| FOX-12 | 503 | 579 |
| RDX | 504 | 858 |
| Toxicity assessment | ||
|---|---|---|
| Compound | EC50 (15 min) | EC50 (30 min) |
| 2 | — | 3.78 |
| FOX-12 | 2.15 | 3.58 |
| RDX | 0.237 | 0.239 |
The toxicity to aquatic life was investigated using the luminescent marine bacterium Vibrio fischeri (see ESI†).32 For the dinitramide 2 and FOX-12 we observed EC50 values of 3.78 and 3.58 g L−1 after an incubation time of 30 min. A compound can be considered as non-toxic if EC50 is >1.00 g L−1. The toxicity test shows the low toxicity of 2 and FOX-12 compared with RDX (Table 3).
Spectroscopy
Compounds 1–13 were characterized by 1H NMR and 13C NMR spectroscopy. Compound 1 shows two broad signals at 5.91 and 5.81 ppm representing two –NH2 groups each. These values match the chemical shifts reported previously (5.90 and 5.80 ppm).9 Carbon resonances of the poorly soluble compound 1 could only be observed in a 13C NMR long-time measurement (pulse delay >2 s, >8000 scans). The resulting spectrum shows two sharp peaks at 155.4 (C–NH2) and 139.4 ppm (C–C).The 1H-NMR spectra of compounds 2–5, 8 and 11–13, which all contain only N-connected protons, each reveals two similar broad signals with chemical shifts of 8.64–8.56 and 6.16–6.08 ppm. With two broad signals at 8.10 and 6.17 ppm, the energetic salt 6 shows chemical shifts that are slightly shifted up-field compared with the signals of most of the compounds. The proton signals of compounds 7 and 9 are slightly shifted up-field to 7.65 and 6.00 ppm and 7.82 and 6.05 ppm, respectively. In comparison, compound 10, which is the 1-methyl derivative of compound 9, shows an even greater chemical shift up-field to 6.89 and 4.64 ppm.
The chemical shifts of the carbon atoms of the cations in compounds 2–13 are slightly shifted up-field compared with the neutral compound 1. The chemical shifts of all carbons in the cations of compounds 2–13 show very sharp signals in a similar range. The carbon of the C–NH2 group shows a chemical shift between 153.6 and 151.9 ppm, whereas the chemical shift of the C–C carbon lies between 138.5 and 137.7 ppm.
IR and Raman spectra for compounds 1–13 were measured and the frequencies were assigned according to commonly observed values.5,33
In the IR spectrum of compound 1, the stretching vibration of the N–H bond is observed between 3500 and 3300 cm−1, whereas the deformation vibration shows a strong band at 1537 cm−1 in the IR spectrum and a very weak band at 1551 cm−1 in the Raman spectrum. The strongest band observed in the IR spectrum is the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretch at 1626 cm−1. Another characteristic band of the triazole ring is observed at 1317 cm−1, representing the C–N stretch. These values are very similar to those reported for substituted 1,2,4-triazoles.34 The vibration of the carbon–amine bond appears at 1085 cm−1 and the C–C vibration of the carbons linking the two rings together occurs at 1022 cm−1. These bands can all be observed at very similar values for the cations of compounds 2–13.
N stretch at 1626 cm−1. Another characteristic band of the triazole ring is observed at 1317 cm−1, representing the C–N stretch. These values are very similar to those reported for substituted 1,2,4-triazoles.34 The vibration of the carbon–amine bond appears at 1085 cm−1 and the C–C vibration of the carbons linking the two rings together occurs at 1022 cm−1. These bands can all be observed at very similar values for the cations of compounds 2–13.
The 13C NMR peaks and the IR and Raman bands of the anions all match the previously published values and are discussed in detail in the ESI.†
Experimental part
The general methods and procedures as well as the synthesis of compounds 3–13 are described in the ESI.†Conclusion
The aromatic, nitrogen-rich 4,4′,5,5′-tetraamino-3,3′-bi-1,2,4-triazole (1) was synthesized from commercially available diaminoguanidine hydrochloride and oxalic acid in polyphosphoric acid. Compound 1 shows an excellent thermal stability in both its neutral (342 °C) and protonated forms. Through simple anion metathesis several new energetic salts (2–13) were obtained and characterized in detail. These energetic ionic derivatives were extensively characterized for their physico-chemical properties (stability, sensitivity, compatibility) and detonation parameters based on their enthalpies of formation calculated using the EXPLO5 computer code. The dinitramide salt 2 has a heat of formation of 301.5 kJ mol−1, a detonation pressure of 338 kbar and a detonation velocity of 9053 m s−1, which are remarkably high compared with other nitrogen-rich dinitramide salts, such as FOX-12. Moreover, dinitramide 2 was measured to be less toxic than RDX in aqueous media. Fundamental compatibility tests demonstrate the compatibility of 2 with TNT.The high decomposition temperature (200 °C, determined by DTA at a heating rate of 5 °C min−1) of dinitramide 2 is better than nearly all the dinitramides reported previously. The high thermal stability of these energetic salts shows the great value of compound 1 over other nitrogen-rich cations, such as guanidine, aminoguanidine or triaminoguanidine, in the synthesis of new ionic energetic materials.
Acknowledgements
Financial support of this work by the Ludwig-Maximilian University of Munich (LMU), the U.S. Army Research Laboratory (ARL) under grant no. W911NF-09-2-0018, the Armament Research, Development and Engineering Center (ARDEC) under grant nos W911NF-12-1-0467 & W911NF-12-1-0468, and the Office of Naval Research (ONR) under grant no. ONR.N00014-12-1-0538 is gratefully acknowledged. The authors acknowledge collaboration with Dr Mila Krupka (OZM Research, Czech Republic) in the development of new testing and evaluation methods for energetic materials and with Dr Muhamed Sucesca (Brodarski Institute, Croatia) in the development of new computational codes to predict the detonation and propulsion parameters of novel explosives. We are indebted to and thank Drs Betsy M. Rice and Brad Forch (ARL, Aberdeen, Proving Ground, MD) for many inspired discussions. The authors want to thank Stefan Huber for measuring the sensitivities, Regina Scharf for measuring the toxicities and Daniel W. Terwilliger for his contributions.Notes and references
- (a) W. E. Bachmann and J. C. Sheehan, J. Am. Chem. Soc., 1949, 71, 1842–1845 CrossRef CAS; (b) E. P. Burrows and E. E. Brueggemann, J. Chromatogr., 1985, 329, 285–289 CrossRef CAS; (c) A. T. Nielsen, A. P. Chafin, S. L. Christian, D. W. Moore, M. P. Nadler, R. A. Nissan, D. J. Vanderah, R. D. Gilardi, C. F. George and J. L. Flippen-Anderson, Tetrahedron, 1998, 54, 11793–11812 CrossRef CAS.
- T. Fendt, N. Fischer, T. M. Klapötke and J. Stierstorfer, Inorg. Chem., 2011, 50, 1447–1458 CrossRef CAS PubMed.
- (a) H. Gao and J. M. Shreeve, Chem. Rev., 2011, 111, 7377–7436 CrossRef CAS PubMed; (b) R. Haiges, S. Schneider, T. Schroer and K. O. Christe, Angew. Chem., 2004, 116, 5027–5032 ( Angew. Chem., Int. Ed. , 2004 , 43 , 4919–4924 ) CrossRef; (c) D. E. Chavez, M. A. Hiskey, D. L. Naud and D. Parrish, Angew. Chem., 2008, 120, 8431–8433 ( Angew. Chem., Int. Ed. , 2008 , 47 , 8307–8309 ) CrossRef; (d) M. B. Talawar, R. Sivabalan, T. Mukundan, H. Muthurajan, A. K. Sikder, B. R. Gandhe and A. Subhananda Rao, J. Hazard. Mater., 2009, 161, 589–607 CrossRef CAS PubMed; (e) O. S. Bushuyev, P. Brown, A. Maiti, R. H. Gee, G. R. Peterson, B. L. Weeks and L. J. Hope-Weeks, J. Am. Chem. Soc., 2012, 134, 1422–1425 CrossRef CAS PubMed; (f) C. Li, L. Liang, K. Wang, C. Bian and J. Zhang, J. Mater. Chem. A, 2014, 2, 18097–18105 RSC.
- (a) D. Fischer, T. Klapötke and J. Stierstorfer, Angew. Chem., 2014, 126, 8311–8314 ( Angew. Chem., Int. Ed. , 2014 , 53 , 8172–8175 ) CrossRef; (b) M. Göbel and T. M. Klapötke, Z. Anorg. Allg. Chem., 2007, 633, 1006–1017 CrossRef; (c) M. Härtel, T. M. Klapötke, D. Piercey and J. Stierstorfer, Z. Anorg. Allg. Chem., 2012, 638, 2008–2014 CrossRef.
- T. M. Klapötke, P. Mayer, C. M. Sabaté, J. M. Welch and N. Wiegand, Inorg. Chem., 2008, 47, 6014–6027 CrossRef PubMed.
- D. E. Chavez, M. A. Hiskey and R. D. Gilardi, Angew. Chem., 2000, 112, 1861–1863 ( Angew. Chem., Int. Ed. , 2000 , 39 , 1791–1793 ) CrossRef.
- (a) A. Hammerl, M. A. Hiskey, G. Holl, T. M. Klapötke, K. Polborn, J. Stierstorfer and J. J. Weigand, Chem. Mater., 2005, 17, 3784–3793 CrossRef CAS; (b) L. Liang, K. Wang, C. Bian, L. Ling and Z. Zhou, Chem.–Eur. J., 2013, 19, 14902–14910 CrossRef CAS PubMed; (c) L. Liang, H. Huang, K. Wang, C. Bian, J. Song, L. Ling, F.- Zhao and Z. Zhou, J. Mater. Chem., 2012, 22, 21954–21964 RSC.
- (a) A. A. Dippold and T. M. Klapötke, J. Am. Chem. Soc., 2013, 135, 9931–9938 CrossRef CAS PubMed; (b) J. Zhang and J. M. Shreeve, J. Am. Chem. Soc., 2014, 136, 4437–4445 CrossRef CAS PubMed; (c) M. Göbel, K. Karaghiosoff, T. M. Klapötke, D. G. Piercey and J. Stierstorfer, J. Am. Chem. Soc., 2010, 132, 17216–17226 CrossRef PubMed; (d) J. Song, Z. Zhou, X. Dong, H. Huang, D. Cao, L. Liang, K. Wang, J. Zhang, F. Chen and Y. Wu, J. Mater. Chem., 2012, 22, 3201–3209 RSC.
- R. Centore, A. Carella and S. Fusco, Struct. Chem., 2011, 22, 1095–1103 CrossRef CAS.
- ESI.†.
- H. Östmark, U. Bemm, A. Lenglet, R. Sandén and N. Wingborg, J. Energ. Mater., 2000, 18, 123–138 CrossRef.
- R. J. Schmidt, J. C. Battaro and P. E. Penwell, The Development of New Protecting/Leaving Groups and Application to the Synthesis of Cage Nitramines, SRI International Project 6654, ADA261496, 1993.
- A. Dippold and T. M. Klapötke, Chem.–Eur. J., 2012, 18, 16742–16753 CrossRef CAS PubMed.
- A. A. Dippold and T. M. Klapötke, Chem.–Asian J., 2013, 8, 1463–1471 CrossRef CAS PubMed.
- A. Rheingold, J. Cronin and T. Brill, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1987, 43, 402–404 CrossRef.
- A. Katrusiak, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1994, 50, 1161–1163 CrossRef.
- S. H. Kim and J. S. Kim, Bull. Korean Chem. Soc., 2013, 34, 2503–2506 CrossRef CAS.
- T. M. Klapötke, A. Preimesser and J. Stierstorfer, Z. Anorg. Allg. Chem., 2012, 638, 1278–1286 CrossRef.
- N. Fischer, D. Fischer, T. M. Klapötke, D. G. Piercey and J. Stierstorfer, J. Mater. Chem., 2012, 22, 20418–20422 RSC.
- N. Fischer, T. Klapötke, M. Reymann and J. Stierstorfer, Eur. J. Inorg. Chem., 2013, 2167–2180 CrossRef CAS.
- J. P. Agrawal, High Energy Materials, Wiley-VCH, Weinheim, 1st edn, 2010, p. 189 Search PubMed.
- K. Shipp, J. Org. Chem., 1964, 29, 2620–2623 CrossRef CAS.
- H. Östmark, A. Helte, T. Carlsson, R. Adolfsson, L. Bodin, C. Eldstäter, H. Edvinsson, J. Lundgreen and H. Örnhed, FOI Technical Report, N-guanylurea dinitramide (FOX-12): properties 2007, FOI-R--2312—SE, Project no. E20503.
- T. M. Klapötke, J. Stierstorfer and A. U. Wallek, Chem. Mater., 2008, 20, 4519–4530 CrossRef.
- D. Fischer, private communication.
- M. Göbel and T. M. Klapötke, Z. Anorg. Allg. Chem., 2007, 633, 1006–1017 CrossRef.
- (a) T. M. Klapötke and J. Stierstorfer, Chem. Phys., 2008, 10, 4340–4346 Search PubMed; (b) T. M. Klapötke and J. Stierstorfer, Eur. J. Inorg. Chem., 2008, 26, 4055–4062 CrossRef; (c) T. M. Klapötke and J. Stierstorfer, Dalton Trans., 2009, 4, 643–653 Search PubMed; (d) T. M. Klapötke, F. A. Martin, N. T. Mayr and J. Stierstorfer, Z. Anorg. Allg. Chem., 2010, 636, 2555–2564 CrossRef; (e) J. C. Oxley, J. L. Smith and H. Chen, Thermochim. Acta, 2002, 384, 91–99 CrossRef CAS.
- U. Teipel, T. Heintz and H. H. Krause, Propellants, Explos., Pyrotech., 2000, 25, 81–85 CrossRef CAS.
- J. Köhler and R. Meyer, Explosivstoffe, Wiley-VCH, Weinheim, 9th edn, 1998, pp. 166–168 Search PubMed.
- M. Sućeska, EXPLO5.06 program, Zagreb, Croatia, 2013 Search PubMed.
- C. Xue, J. Sun, B. Kang, Y. Liu, X. Liu, G. Song and Q. Xue, Propellants, Explos., Pyrotech., 2010, 35, 333–338 CrossRef CAS.
- G. I. Sunahara, S. Dodard, M. Sarrazin, L. Paquet, G. Ampleman, S. Thiboutot, J. Hawari and A. Y. Renoux, Ecotoxicol. Environ. Saf., 1998, 39, 185–194 CrossRef CAS PubMed.
- (a) M. Hesse, H. Meier and B. Zeeh, Spektroskopische Methoden in der Organischen Chemie, Thieme, Stuttgart, New York, 7th edn, 2005 Search PubMed; (b) G. Socrates, Infrared and Raman Characteristic Group Frequencies: Tables and Charts, John Wiley & Sons, Chichester, 3rd edn, 2004 Search PubMed.
- Y. Murtim, R. Agnihotri and D. Pathak, Am. J. Chem., 2011, 1, 42–46 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: (1) Materials and methods; (2) experimental work; (3) X-ray diffraction; (4) electron microscopy; (5) explosive performance; (6) toxicity assessment; (7) spectroscopy; cif files. CCDC 1029053–1029066. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4ta05964f |
| This journal is © The Royal Society of Chemistry 2015 |