Origin of non-SEI related coulombic efficiency loss in carbons tested against Na and Li†
Abstract
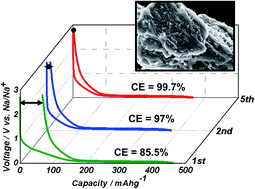
Partially ordered but not graphitized carbons are widely employed for sodium and lithium ion battery (NIB and LIB) anodes, either in their pure form or as a secondary supporting phase for oxides, sulfides and insertion electrodes. These “pseudographitic” materials ubiquitously display a poor initial coulombic efficiency (CE), which has been historically attributed to solid electrolyte interface (SEI) formation on their large surface areas (up to ∼2500 m2 g−1). Here we identify the other sources CE loss by examining a pseudographitic carbon with a state-of-the-art capacity (>350 mA h g−1 for NIB, >800 mA h g−1 for LIB), but with a purposely designed low surface area (14.5 m2 g−1) that disqualifies SEI from having a substantial role. During the initial several (<5) cycles both Na and Li are irreversibly trapped in the bulk, with the associated CE loss occurring at higher desodiation/delithiation voltages. We measure a progressively increasing graphene interlayer spacing and a progressively increasing Raman G band intensity, indicating that the charge carriers become trapped not only at the graphene defects but also between the graphene planes hence causing them to both dilate and order. For the case of Li, we also unambiguously detected irreversible metal underpotential deposition (“nanoplating”) within the nanopores at roughly below 0.2 V. It is expected that in conventional high surface area carbons these mechanisms will be a major contributor to CE loss in parallel to classic SEI formation. Key implications to emerge from these findings are that improvements in early cycling CE may be achieved by synthesizing pseudographitic carbons with lower levels of trapping defects, but that for LIBs the large cycle 1 CE loss may be unavoidable if highly porous structures are utilized.

 Please wait while we load your content...
Please wait while we load your content...