Synthesis of Cu9S8/carbon nanotube nanocomposites with high electrocatalytic activity for the oxygen reduction reaction†
Abstract
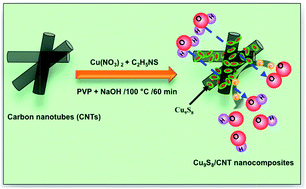
We have synthesized Cu9S8/carbon nanotube (CNT) nanocomposites (NCs) with high electrocatalytic activity for direct methanol fuel cells (DMFCs). Cu9S8/CNT NCs are prepared from Cu(NO3)2, CNTs, and thioacetamide in the presence of poly(vinylpyrrolidone) under alkaline conditions. There are Cu9S8 nanoparticles (diameter: 50 ± 6 nm) and aggregates on the surfaces of CNTs. The as-prepared Cu9S8/CNT NC modified electrodes provide a four-electron pathway for the oxygen reduction reaction (ORR) in alkaline media. Three representative Cu9S8/CNT electrodes (mass loading: 1.63 mg cm−2) provide a mean limiting current density of 3.43 ± 0.03 mA cm−2 (each with three measurements) at a constant scan rate of 1 mV s−1 and a rotation rate of 3600 rpm. At a constant potential of −0.5 V, the kinetic rate constant for the Cu9S8/CNT is 2.82 × 10−2 cm s−1, revealing higher activity of the Cu9S8/CNT electrodes in the ORR. The Cu9S8/CNT relative to Pt/C electrodes is more tolerant against methanol and carbon monoxide poisoning. These low-cost, stable, and highly active Cu9S8/CNT electrodes have great potential for use in DMFCs.

 Please wait while we load your content...
Please wait while we load your content...