Open Access Article
Open Access ArticleA highly permeable polyimide with enhanced selectivity for membrane gas separations†
Yulia
Rogan
a,
Richard
Malpass-Evans
a,
Mariolino
Carta
b,
Michael
Lee
b,
Johannes C.
Jansen
*c,
Paola
Bernardo
c,
Gabriele
Clarizia
c,
Elena
Tocci
c,
Karel
Friess
d,
Marek
Lanč
d and
Neil B.
McKeown
*b
aSchool of Chemistry, Cardiff University, Cardiff, CF10 2AT, UK
bEaStCHEM and School of Chemistry, University of Edinburgh, West Mains Road, Edinburgh EH9 3JJ, Scotland, UK. E-mail: neil.mckeown@ed.ac.uk
cInstitute on Membrane Technology, ITM-CNR, c/o University of Calabria, Via P. Bucci 17/C, 87036 Rende, CS, Italy
dInstitute of Chemical Technology, Department of Physical Chemistry, Technická 5, Prague 6, 166 28, Czech Republic
First published on 20th February 2014
Abstract
A highly gas permeable polyimide with improved molecular sieving properties is produced by using a bisanhydride monomer based on the rigid ethanoanthracene unit. The polymer (PIM-PI-EA) demonstrates enhanced selectivity for gas separations so that its gas permeability data lie above the 2008 Robeson upper bounds for the important O2–N2, H2–N2, CO2–CH4 and CO2–N2 gas pairs.
Polymers that can be processed from solution to form microporous solids are of growing interest for use as highly permeable membranes for gas separations including O2 or N2 enrichment from air, natural gas upgrading (predominantly removing CO2 from CH4), and hydrogen recovery from ammonia manufacture (separating H2 from N2).1–3 Polymers currently used as membranes for commercial gas separation, such as the polyimide Matrimid,4 generally demonstrate high selectivity but low permeability thus limiting industrial membrane applications to small and medium-scale gas separations. In contrast, highly permeable polymers have potential for large-scale separations but only if selectivity can be enhanced.3 However, for the separation of two gases (x and y), there is a well-established empirical trade-off between permeability (Px) and selectivity (αxy = Px/Py) that was quantified by Robeson initially in 1991 (ref. 5) and then updated in 2008.6 Based on the permeability data for a large number of polymers, Robeson identified an “upper bound” in plots of log
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pxversus log
Pxversus log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) αxy for each gas pair of interest. The position of a polymer's gas permeability data relative to the relevant upper bound is used as the universal performance indicator for assessing its potential for the separation of two gases.
αxy for each gas pair of interest. The position of a polymer's gas permeability data relative to the relevant upper bound is used as the universal performance indicator for assessing its potential for the separation of two gases.
Robeson noted6 that each of the upper bounds are populated by data from glassy polymers with rigid chains that promote diffusivity selectivity – i.e. the preferential transport of lighter gas molecules of smaller kinetic diameters (e.g. He = 2.65; H2 = 2.80; CO2 = 3.3; O2 = 3.45 Å) over that of larger molecules (e.g. N2 = 3.64; CH4 = 3.87 Å). Hence it was predicted that greater size selectivity could be obtained by increasing polymer chain rigidity whereas high gas permeability relies on large inter-chain separation.7 Polymers of Intrinsic Microporosity (PIMs), such as the archetypal PIM-1 (Fig. 1a), fulfil both of these design criteria by possessing a rigid chain structure within which motion is restricted and sites of contortion, often provided by spirobisindan (SBI) units, to prohibit space efficient packing and generate high free volume.2,8–10 Therefore, permeability data for PIMs generally lie over the 1991 upper bound for most gas pairs10,11 and some approach, or even exceed, the 2008 upper bounds (Fig. 2).12,13 Similarly, microporous polyimides based on the SBI unit also demonstrate high permeability with modest selectivity (e.g. PIM-PI-SBI; Fig. 1b).14,15
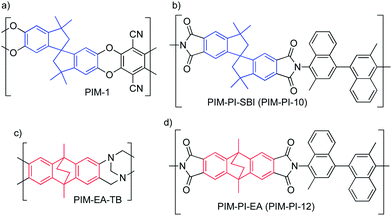 | ||
| Fig. 1 The molecular structures of (a) PIM-1; (b) PIM-PI-SBI; (c) PIM-EA-TB and (d) PIM-PI-EA. Spirobisindan (SBI) is blue, ethanoanthracene (EA) is red. | ||
 | ||
Fig. 2 Robeson plots for (a) O2–N2; (b) H2–N2; (c) CO2–CH4 and (d) CO2–N2 gas pairs showing the data  for methanol treated 72 μm film of PIM-PI-EA. The data point at lower permeability and higher selectivity in each plot is for the same film aged for 273 days. Data points for methanol treated 72 μm film of PIM-PI-EA. The data point at lower permeability and higher selectivity in each plot is for the same film aged for 273 days. Data points  are for other PIM-PIs with the arrows starting from the data point are for other PIM-PIs with the arrows starting from the data point  for PIM-PI-SBI (ESI Table 1†) representing the enhancement in performance on exchanging the SBI for EA units. The gray and black lines represent the 1991 and 2008 upper bounds, respectively. Data points ◇ are for PIM-EA-TB. Also shown are data points × of other PIMs. PIMs that demonstrate data that cross various upper bounds are reported in ref. 9, 12, 13 and 16. for PIM-PI-SBI (ESI Table 1†) representing the enhancement in performance on exchanging the SBI for EA units. The gray and black lines represent the 1991 and 2008 upper bounds, respectively. Data points ◇ are for PIM-EA-TB. Also shown are data points × of other PIMs. PIMs that demonstrate data that cross various upper bounds are reported in ref. 9, 12, 13 and 16. | ||
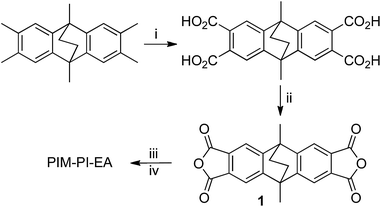
Recently, we demonstrated that the polymerisation of 2,6(7)-diamino-9,10-dimethylethanoanthracene by the formation of linking groups based on Tröger's base (TB) provides a highly permeable polymer (PIM-EA-TB; Fig. 1c) with remarkably high gas selectivities so that its data lie well above the 2008 upper bounds for O2–N2 (Fig. 2a), H2–N2 (Fig. 2b), H2–CH4 and H2–CO2.16 The exceptional performance of PIM-EA-TB was attributed to enhanced diffusivity selectivity as a result of its highly shape-persistent structure provided by the bridged bicyclic ethanoanthracene (EA) component as compared to the more flexible SBI unit.16 Hence it is important to establish whether the exchange of SBI components for EA units in other types of PIMs may also result in enhanced size-selectivity for gas separations and provides a general concept for improving polymers for gas separations.17 Here we validate this concept by the synthesis of an EA-based polyimide (PIM-PI-EA; Fig. 1d), which has a structure that allows the direct comparison of its performance with a recently reported highly permeable SBI-based polyimide (PIM-PI-SBI; Fig. 1b).14
The required novel EA-based bisanhydride monomer 1 was prepared from 2,3,6,7,9,10-hexamethylethanoanthracene18 and reacted with commercially available 3,3′-dimethylnaphthidine, a monomer of previous utility for preparing highly gas permeable polyimides (Scheme 1 and ESI†).14,15 The resulting polymer, PIM-PI-EA, was obtained in 88% yield and proved freely soluble in chloroform and THF, which facilitated its characterisation by 1H NMR spectroscopy and Gel permeation chromatography (GPC). The latter indicated that PIM-PI-EA was of very high average molecular mass (Mw = 340![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000; Mn = 110
000; Mn = 110![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1) as calibrated against polystyrene standards. Consequently, cast films of PIM-PI-EA from chloroform solution proved robust and suitable for gas permeability measurements. Nitrogen adsorption isotherms for a powdered sample of PIM-PI-EA at 77 K showed significant uptake at low relative pressures indicative of intrinsic microporosity and provided an apparent BET surface area of 616 m2 g−1, which is lower than that of PIM-PI-SBI (699 m2 g−1).
000 g mol−1) as calibrated against polystyrene standards. Consequently, cast films of PIM-PI-EA from chloroform solution proved robust and suitable for gas permeability measurements. Nitrogen adsorption isotherms for a powdered sample of PIM-PI-EA at 77 K showed significant uptake at low relative pressures indicative of intrinsic microporosity and provided an apparent BET surface area of 616 m2 g−1, which is lower than that of PIM-PI-SBI (699 m2 g−1).
The gas permeability data for a 72 μm thick film of PIM-PI-EA are provided in Table 1 and the equivalent data for PIM-PI-SBI, PIM-1 and PIM-EA-TB are given in the ESI.†14 Prior to analysis the film of PIM-PI-EA was treated by immersion in methanol as this is known to reverse the effects of physical ageing for glassy polymers and also removes the last residues of the casting solvent.9,12,16 Hence, this treatment allows a direct comparison between the gas permeabilities of different polymers.
| N2 | O2 | CO2 | CH4 | H2 | He | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a Units = barrer. b α = (Px/PN2). c Units = 10−12 m2 s−1. d Units = cm3 cm−3 bar−1. e For He and H2 the time lag is too short (<1 s) for determination of D with error <10%, and the value of D is the minimum limit. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| P x | 369 (131) | 1380 (659) | 7340 (3230) | 457 (156) | 4230 (2860) | 1580 (1130) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| α | — (—) | 3.7 (5.0) | 19.9 (24.6) | 1.2 (1.2) | 11.5 (21.8) | 4.3 (8.6) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| D x | 84 (32) | 270 (144) | 95 (48) | 24 (8.4) | ≥3360e (3581) | ≥5070e (5740) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| D x/DN2 | — | 3.2 (4.8) | 1.1 (1.6) | 0.29 (0.28) | 40e (120) | 60e (191) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| S x | 3.29 (3.3) | 3.83 (3.4) | 57.8 (50.1) | 14.2 (13.5) | ≤0.94 (0.60) | ≤0.23 (0.15) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| S x/SN2 | — | 1.2 (1.1) | 17.6 (15.2) | 4.3 (4.1) | 0.29 (0.18) | 0.07 (0.04) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The order of gas permeabilities for the freshly methanol treated film of PIM-PI-EA is CO2 > H2 > He > O2 > CH4 > N2. This differs from most other PIMs including PIM-1 and PIM-PI-SBI in that He is faster than O2. It is notable that the value for N2 permeability through PIM-PI-EA is very similar to that of PIM-PI-SBI, whereas gases composed of smaller molecules (He, H2, CO2 and O2) are transported faster and CH4, composed of larger molecules, is transported significantly slower through PIM-PI-EA relative to PIM-PI-SBI. These results validate the efficacy of the rigid ethanoanthracene unit as a component for inducing high gas selectivity via molecular sieving.
Importantly, the data points for PIM-PI-EA are above the Robeson upper bounds for O2–N2 (Fig. 2a), H2–N2 (Fig. 2b), CO2–CH4 (Fig. 2c), CO2–N2 (Fig. 2d) and H2–CH4 gas pairs, in contrast to those of PIM-PI-SBI, which fall below these upper bounds. The results for the CO2–CH4 and CO2–N2 gas pairs are particularly encouraging, as the equivalent data for the ethanoanthracene-containing PIM-EA-TB are anomalously poor and fall below the relevant upper bounds.16
Physical ageing (i.e. loss of free volume and gas permeability over time) is a general feature of glassy polymers19 and is observed for PIM-PI-EA (Table 1). However, the loss of permeability due to aging is matched by a commensurate increase in selectivity so that the data points for the polymer aged for 273 days all lie above the upper bounds for O2–N2 (Fig. 2a), H2–N2 (Fig. 2b), CO2–CH4 (Fig. 2c), CO2–N2 (Fig. 2d) and H2–CH4 gas pairs. For the O2–N2 gas pair, the selectivity in favour of O2 of 5.0 for a value of PO2 greater than 600 barrer is exceptional.
According to the solution-diffusion model, the permeability of a gas through a polymer is dependent on both its diffusivity (Dx) and its solubility (Sx) (i.e., Px = DxSx).20 The enhanced permeability of He, H2 and O2 is attributable to greater diffusivity (Dx) within PIM-PI-EA, whereas, the enhanced permeability of CO2 appears to be related to its greater solubility in PIM-PI-EA as compared to PIM-PI-SBI (Table 1 and ESI Table 1†), as demonstrated by CO2 sorption isotherms measured at 298 K. (ESI Fig. 1a†). The solubility of CH4 within PIM-PI-EA and PIM-PI-SBI (ESI Fig. 1b†) is very similar. Therefore, the impressive potential of PIM-PI-EA for the commercially important CO2–CH4 separation is due to both enhanced diffusivity selectivity and solubility selectivity. CO2 sorption is higher in PIM-PI-SBI than in PIM-1, but not as high as in PIM-EA-TB, where the tertiary amine groups of the Tröger's base appear to contribute to the affinity for CO2 (ESI Fig. 1a†).21
Molecular modelling, which is the only technique which can visualize the free volume distribution, supports these findings.22,23 The high rigidity of the polymer structure is reflected in the difficulty to pack the polymer models. Fig. 3 shows the free volume in a PIM-PI-EA model, packed and equilibrated at the measured film density of 1.1 g cm−3. The small o-positronium probe gains access to the highly interconnected void structure. The H2 molecule is able to access more free volume than the larger N2. For H2 the FV is also more interconnected than for the N2 probe. This explains the higher diffusivity of H2 as compared to N2 found experimentally (Table 1).
From a fundamental perspective, this study provides a direct comparison between structurally related polyimides PIM-PI-EA and PIM-PI-SBI and demonstrates the advantage of the rigid bridged bicyclic ethanoanthracene over the more flexible and often used spirobisindane for enhancing diffusivity selectivity.16,22 In addition, PIM-PI-EA has gas permeability data that lie well above the Robeson upper bounds for important gas pairs including CO2–CH4, of interest for natural gas upgrading, and CO2–N2, of interest for post-combustion carbon capture applications.24 This significant enhancement of gas selectivity for highly permeable polyimides is of particular importance as polyimides are the most studied class of polymer for membrane applications.25 Therefore, methodologies that have been developed for polyimide membrane technology (e.g. cross-linking strategies)26 can also be applied to PIM-PI-EA, thus making it a strong candidate for exploitation as a membrane material.
Acknowledgements
European Community's Seventh Framework Programme (FP7/2007-2013) under grant agreement no. NMP3-SL-2009-228631 “DoubleNanoMem”, the Italian Programma Operativo Nazionale Ricerca e Competitività 2007–2013, project PON01_01840 “MicroPERLA”. The authors are also thankful for financial support of the Grant Agency of Czech Republic (Grants no. 106/10/1194).Notes and references
- (a) P. Bernardo, E. Drioli and G. Golemme, Ind. Eng. Chem. Res., 2009, 48, 4638 CrossRef CAS; (b) D. E. Sanders, Z. P. Smith, R. Guo, L. M. Robeson, J. E. McGrath, D. R. Paul and B. D. Freeman, Polymer, 2013, 54, 4729 CrossRef CAS PubMed; (c) Y. Yampolskii, Macromolecules, 2012, 45, 3298 CrossRef CAS; (d) J. K. Adewole, A. L. Ahmad, S. Ismail and C. P. Leo, Int. J. Greenhouse Gas Control, 2013, 17, 46 CrossRef CAS PubMed; (e) R. W. Baker and K. Lokhandwala, Ind. Eng. Chem. Res., 2008, 47, 2109 CrossRef CAS; (f) C. A. Scholes, G. W. Stevens and S. E. Kentish, Fuel, 2012, 96, 15 CrossRef CAS PubMed; (g) R. S. Murali, T. Sankarshana and S. Sridhar, Sep. Purif. Rev., 2013, 42, 130 CrossRef CAS.
- N. B. McKeown and P. M. Budd, in Encyclopedia of Membrane Science and Technology, ed. E. M. V. Koek and V. V. Tarabara, John Wiley & Sons, Inc., 2013, vol. 2, p. 781 Search PubMed.
- P. M. Budd and N. B. McKeown, Polym. Chem., 2010, 1, 63 RSC.
- (a) W. F. Yong, F. Y. Li, Y. C. Xiao, T. S. Chung and Y. W. Tong, J. Membr. Sci., 2013, 443, 156 CrossRef CAS PubMed; (b) Y. Huang and D. R. Paul, Ind. Eng. Chem. Res., 2007, 46, 2342 CrossRef CAS.
- L. M. Robeson, J. Membr. Sci., 1991, 62, 165 CrossRef CAS.
- L. M. Robeson, J. Membr. Sci., 2008, 320, 390 CrossRef CAS PubMed.
- (a) B. D. Freeman, Macromolecules, 1999, 32, 375 CrossRef CAS; (b) L. M. Robeson, B. D. Freeman, D. R. Paul and B. W. Rowe, J. Membr. Sci., 2009, 341, 178 CrossRef CAS PubMed.
- (a) N. B. McKeown and P. M. Budd, Macromolecules, 2010, 43, 5163 CrossRef CAS; (b) N. B. McKeown and P. M. Budd, Chem. Soc. Rev., 2006, 35, 675 RSC; (c) P. M. Budd, B. S. Ghanem, S. Makhseed, N. B. McKeown, K. J. Msayib and C. E. Tattershall, Chem. Commun., 2004, 230 RSC; (d) P. M. Budd, E. S. Elabas, B. S. Ghanem, S. Makhseed, N. B. McKeown, K. J. Msayib, C. E. Tattershall and D. Wang, Adv. Mater., 2004, 16, 456 CrossRef CAS; (e) M. Carta, K. J. Msayib, P. M. Budd and N. B. McKeown, Org. Lett., 2008, 10, 2641 CrossRef CAS PubMed; (f) A. V. Maffei, P. M. Budd and N. B. McKeown, Langmuir, 2006, 22, 4225 CrossRef CAS PubMed.
- P. M. Budd, N. B. McKeown, B. S. Ghanem, K. J. Msayib, D. Fritsch, L. Starannikova, N. Belov, O. Sanfirova, Y. P. Yampol'skii and V. Shantarovich, J. Membr. Sci., 2008, 325, 851 CrossRef CAS PubMed.
- P. M. Budd, K. J. Msayib, C. E. Tattershall, B. S. Ghanem, K. J. Reynolds, N. B. McKeown and D. Fritsch, J. Membr. Sci., 2005, 251, 263 CrossRef CAS PubMed.
- B. S. Ghanem, N. B. McKeown, P. M. Budd and D. Fritsch, Macromolecules, 2008, 41, 1640 CrossRef CAS.
- C. G. Bezzu, M. Carta, A. Tonkins, J. C. Jansen, P. Bernardo, F. Bazzarelli and N. B. McKeown, Adv. Mater., 2012, 24, 5930 CrossRef CAS PubMed.
- N. Du, H. B. Park, G. P. Robertson, M. M. Dal-Cin, T. Visser, L. Scoles and M. D. Guiver, Nat. Mater., 2011, 10, 372 CrossRef CAS PubMed.
- Y. Rogan, L. Starannikova, V. Ryzhikh, Y. Yampolskii, P. Bernardo, F. Bazzarelli, J. C. Jansen and N. B. McKeown, Polym. Chem., 2013, 4, 3813 RSC.
- (a) B. S. Ghanem, N. B. McKeown, P. M. Budd, N. M. Al-Harbi, D. Fritsch, K. Heinrich, L. Starannikova, A. Tokarev and Y. Yampolskii, Macromolecules, 2009, 42, 7881 CrossRef CAS; (b) B. S. Ghanem, N. B. McKeown, P. M. Budd and D. Fritsch, Adv. Mater., 2008, 20, 2766 CrossRef CAS.
- M. Carta, R. Malpass-Evans, M. Croad, J. C. Jansen, P. Bernardo, F. Bazzarelli and N. B. McKeown, Science, 2013, 339, 303 CrossRef CAS PubMed.
- A PIM derived from 2,3,6,7-tetrahydroxyethanoanthracene and prepared using the same dioxane-forming polymerisation reaction with 2,3,5,6-tetrafluoroterephthalonitrile as is used for making PIM-1 proved to be insoluble although co-polymer has been investigated. See (a) T. Emmler, K. Heinrich, D. Fritsch, P. M. Budd, N. Chaukura, D. Ehlers, K. Ratzke and F. Faupel, Macromolecules, 2010, 43, 6075 CrossRef CAS; (b) D. Fritsch, G. Bengtson, M. Carta and N. B. McKeown, Macromol. Chem. Phys., 2011, 212, 1137 CrossRef CAS.
- L. R. Barclay and R. A. Chapman, Can. J. Chem., 1965, 43, 1754 CrossRef CAS.
- (a) Y. Huang and D. R. Paul, Polymer, 2004, 45, 8377 CrossRef CAS PubMed; (b) M. S. McCaig and D. R. Paul, Polymer, 2000, 41, 629 CrossRef CAS.
- J. G. Wijmans and R. W. Baker, J. Membr. Sci., 1995, 107, 1 CrossRef CAS.
- (a) A. Del Regno, A. Gonciaruk, L. Leay, M. Carta, M. Croad, R. Malpass-Evans, N. B. McKeown and F. Siperstein, Ind. Eng. Chem. Res., 2013, 52, 16939 CrossRef CAS; (b) X. Zhu, D.-T. Chi-Linh, C. R. Murdock, K. M. Nelson, C. Tian, S. Brown, S. M. Mahurin, D. M. Jenkins, J. Hu, B. Zhao, H. Liu and S. Dai, ACS Macro Lett., 2013, 2, 660 CrossRef CAS.
- M. Heuchel, D. Fritsch, P. M. Budd, N. B. McKeown and D. Hofmann, J. Membr. Sci., 2008, 318, 84 CrossRef CAS PubMed.
- G. S. Larsen, P. Lin, K. E. Hart and C. M. Colina, Macromolecules, 2011, 44, 6944 CrossRef CAS.
- N. Y. Du, H. B. Park, M. M. Dal-Cin and M. D. Guiver, Energy Environ. Sci., 2012, 5, 7306 CAS.
- (a) D.-J. Liaw, K.-L. Wang, Y.-C. Huang, K.-R. Lee, J.-Y. Lai and C.-S. Ha, Prog. Polym. Sci., 2012, 37, 907 CrossRef CAS PubMed; (b) Y. Xiao, B. T. Low, S. S. Hosseini, T. S. Chung and D. R. Paul, Prog. Polym. Sci., 2009, 34, 561 CrossRef CAS PubMed; (c) A. Y. Alentev, I. A. Ronova, B. V. Shchukin and Y. P. Yampolskii, Polym. Sci., Ser. A, 2007, 49, 217 CrossRef; (d) R. M. Huertas, A. Tena, A. E. Lozano, J. de Abajo, J. G. de la Campa and E. M. Maya, Polym. Degrad. Stab., 2013, 98, 743 CrossRef CAS PubMed.
- K. Vanherck, G. Koeckelberghs and I. F. J. Vankelecom, Prog. Polym. Sci., 2013, 38, 874 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details for synthesis, characterisation and modeling. Also sorption isotherms for CO2, CH4 and N2 and comparative permeability data for PIM-1, PIM-PI-SBI and PIM-EA-TB. See DOI: 10.1039/c4ta00564c |
| This journal is © The Royal Society of Chemistry 2014 |